Equilibrium Calculations (13.4)
Learning Objectives
By the end of this section, you will be able to:
- Identify the changes in concentration or pressure that occur for chemical species in equilibrium systems
- Calculate equilibrium concentrations or pressures and equilibrium constants, using various algebraic approaches
Having covered the essential concepts of chemical equilibria in the preceding sections of this chapter, this final section will demonstrate the more practical aspect of using these concepts and appropriate mathematical strategies to perform various equilibrium calculations. These types of computations are essential to many areas of science and technology—for example, in the formulation and dosing of pharmaceutical products. After a drug is ingested or injected, it is typically involved in several chemical equilibria that affect its ultimate concentration in the body system of interest. Knowledge of the quantitative aspects of these equilibria is required to compute a dosage amount that will solicit the desired therapeutic effect.
Many of the useful equilibrium calculations that will be demonstrated here require terms representing changes in reactant and product concentrations. These terms are derived from the stoichiometry of the reaction, as illustrated by decomposition of ammonia:
2NH3(g) ⇌ N2(g) + 3H2(g)
As shown earlier in this chapter, this equilibrium may be established within a sealed container that initially contains either NH3 only, or a mixture of any two of the three chemical species involved in the equilibrium. Regardless of its initial composition, a reaction mixture will show the same relationships between changes in the concentrations of the three species involved, as dictated by the reaction stoichiometry (see also the related content on expressing reaction rates in the chapter on kinetics). For example, if the nitrogen concentration increases by an amount x:
Δ[N2] = + x
the corresponding changes in the other species concentrations are:
Δ[H2] = Δ[N2][latex]( \frac{\text{3 mol}H_{2})}{\text{1 mol} N_{2}} )[/latex] = +3x
Δ[NH3] = -Δ[N2][latex]( \frac{\text{2 mol} NH_{3}}{\text{1 mol} N_{2}} )[/latex] = -2x
where the negative sign indicates a decrease in concentration.
Example 13.6
Determining Relative Changes in Concentration
Derive the missing terms representing concentration changes for each of the following reactions.
(a) C2H2(g) + 2Br2(g) ⇌ C2H2Br4(g)
x ___ ___
(b) I2(aq) + I−(aq) ⇌ I3−(aq)
___ ___ x
(c) C3H8(g) + 5O2(g) ⇌ 3CO2(g) + 4H2O(g)
x ___ ____ ____
Solution
(a) C2H2(g) + 2Br2(g) ⇌ C2H2Br4(g)
x 2x -x
(b) I2(aq) + I−(aq) ⇌ I3−(aq)
-x -x x
(c) C3H8(g) + 5O2(g) ⇌ 3CO2(g) + 4H2O(g)
x 5x -3x -4x
Check Your Learning
Complete the changes in concentrations for each of the following reactions:
(a) 2SO2(g) + O2(g) ⇌ 2SO3(g)
___ x ___
(b) C4H8(g) ⇌ 2C2H4(g)
___ -2x
(c) 4NH3(g) + 7H2O(g) ⇌ 4NO2(g) + 6H2O(g)
___ ___ ___ ___
Answer: (a) 2x, x, −2x; (b) x, −2x; (c) 4x, 7x, −4x, −6x or −4x, −7x, 4x, 6x
Calculation of an Equilibrium Constant
The equilibrium constant for a reaction is calculated from the equilibrium concentrations (or pressures) of its reactants and products. If these concentrations are known, the calculation simply involves their substitution into the K expression, as was illustrated by Example 13.2. A slightly more challenging example is provided next, in which the reaction stoichiometry is used to derive equilibrium concentrations from the information provided. The basic strategy of this computation is helpful for many types of equilibrium computations and relies on the use of terms for the
reactant and product concentrations initially present, for how they change as the reaction proceeds, and for what they are when the system reaches equilibrium. The acronym ICE is commonly used to refer to this mathematical approach, and the concentrations terms are usually gathered in a tabular format called an ICE table.
Example 13.7
Calculation of an Equilibrium Constant
Iodine molecules react reversibly with iodide ions to produce triiodide ions.
I2(aq) + I−(aq) ⇌ I3−(aq)
If a solution with the concentrations of I2 and I− both equal to 1.000 × 10−3 M before reaction gives an equilibrium concentration of I2 of 6.61 × 10−4 M, what is the equilibrium constant for the reaction?
Solution
To calculate the equilibrium constants, equilibrium concentrations are needed for all the reactants and products:
Kc = [latex]\frac{[\text{I}^{3-}]}{[I_2][I^-]}[/latex]
Provided are the initial concentrations of the reactants and the equilibrium concentration of the product. Use this information to derive terms for the equilibrium concentrations of the reactants, presenting all the information in an ICE table.
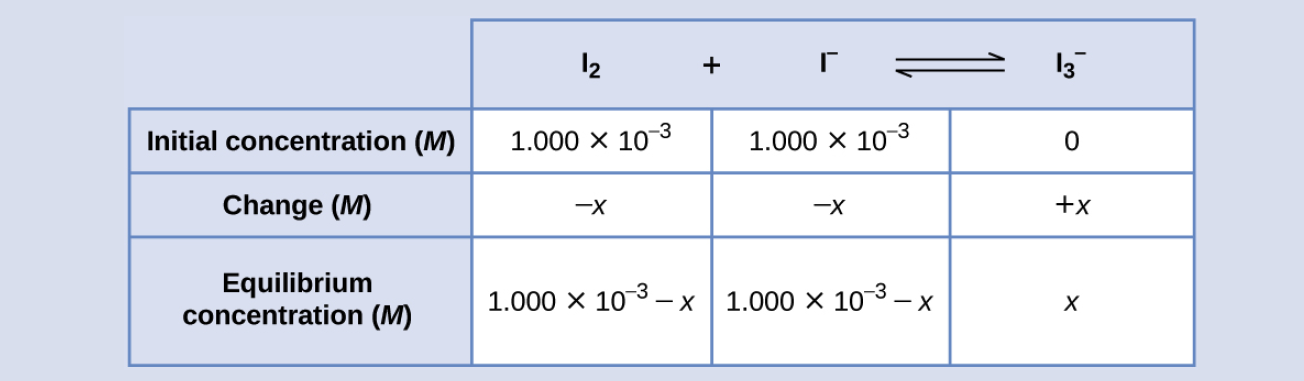
At equilibrium the concentration of I2 is 6.61 × 10−4 M so that
1.000 × 10−3 − x = 6.61 × 10−4
x = 1.000 × 10−3 − 6.61 × 10−4
= 3.39 × 10−4 M
The ICE table may now be updated with numerical values for all its concentrations:
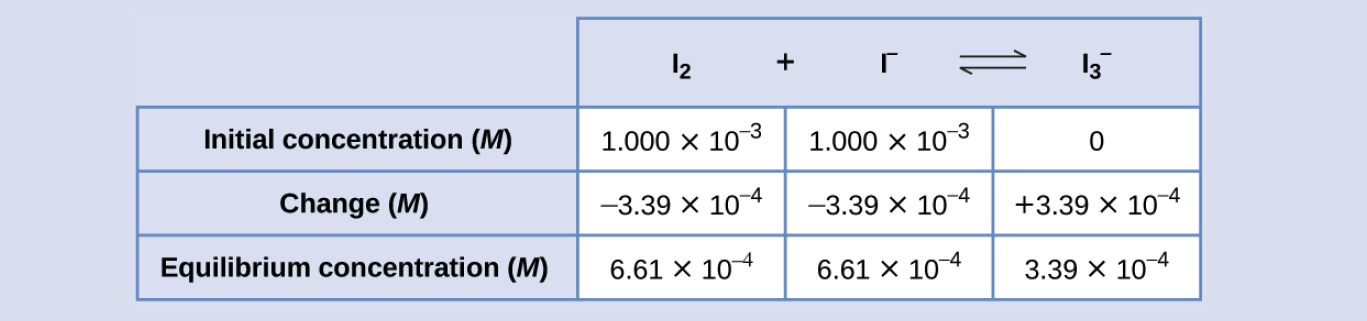
Finally, substitute the equilibrium concentrations into the K expression and solve:
Kc = [latex]\frac{[I_{3}^-]}{[I_2][I^-]}[/latex]
= [latex]\frac{3.39 \times 10^{-4} M}{(6.61 \times 10^{-4} M)(6.61 \times 10^{-4} M)}[/latex] = 776
Check Your Learning
Ethanol and acetic acid react and form water and ethyl acetate, the solvent responsible for the odor of some nail polish removers.
C2H5OH + CH3CO2H ⇌ CH3 CO2C2H5 + H2O
When 1 mol each of C2H5OH and CH3CO2H are allowed to react in 1 L of the solvent dioxane, equilibrium is established when 1/3 mol of each of the reactants remains. Calculate the equilibrium constant for the reaction. (Note: Water is a solute in this reaction.)
Answer: Kc = 4
Calculation of a Missing Equilibrium Concentration
When the equilibrium constant and all but one equilibrium concentration are provided, the other equilibrium concentration(s) may be calculated. A computation of this sort is illustrated in the next example exercise.
Example 13.8
Calculation of a Missing Equilibrium Concentration
Nitrogen oxides are air pollutants produced by the reaction of nitrogen and oxygen at high temperatures. At 2000 °C, the value of the Kc for the reaction, N2(g) + O2(g) ⇌ 2NO(g), is 4.1 × 10−4. Calculate the equilibrium concentration of NO(g) in air at 1 atm pressure and 2000 °C. The equilibrium concentrations of N2 and O2 at this pressure and temperature are 0.036 M and 0.0089 M, respectively.
Solution
Substitute the provided quantities into the equilibrium constant expression and solve for [NO]:
Kc = [latex]\frac{[NO]^2}{[N_2][O_2]}[/latex]
[NO]2 = Kc[N2][O2]
[NO] = [latex]\sqrt{K_{c}[N_{2}][O_{2}]}[/latex]
= [latex]\sqrt{(4.1 \times 10^{-4})(0.036)(0.0089)}[/latex]
= [latex]\sqrt{1.31 \times 10^{-7}}[/latex]
= [latex]3.6 \times 10^{-4}[/latex]
Thus [NO] is 3.6 × 10−4 mol/L at equilibrium under these conditions.
To confirm this result, it may be used along with the provided equilibrium concentrations to calculate a value for K:
Kc = [latex]\frac{[NO]^{2}}{[N_{2}][O_{2}]}[/latex]
= [latex]\frac{(3.6 \times 10^{-4})^2}{(0.036)(0.0089)}[/latex]
= [latex]4.0 \times 10^{-4}[/latex]
This result is consistent with the provided value for K within nominal uncertainty, differing by just 1 in the least significant digit’s place.
Check Your Learning
The equilibrium constant Kc for the reaction of nitrogen and hydrogen to produce ammonia at a certain temperature is 6.00 × 10−2. Calculate the equilibrium concentration of ammonia if the equilibrium concentrations of nitrogen and hydrogen are 4.26 M and 2.09 M, respectively.
Answer: 1.53 mol/L
Calculation of Equilibrium Concentrations from Initial Concentrations
Perhaps the most challenging type of equilibrium calculation can be one in which equilibrium concentrations are derived from initial concentrations and an equilibrium constant. For these calculations, a four-step approach is typically useful:
- Identify the direction in which the reaction will proceed to reach equilibrium.
- Develop an ICE table.
- Calculate the concentration changes and, subsequently, the equilibrium concentrations.
- Confirm the calculated equilibrium concentrations.
The last two example exercises of this chapter demonstrate the application of this strategy.
Example 13.9
Calculation of Equilibrium Concentrations
Under certain conditions, the equilibrium constant Kc for the decomposition of PCl5(g) into PCl3(g) and Cl2(g) is 0.0211. What are the equilibrium concentrations of PCl5, PCl3, and Cl2 in a mixture that initially contained only PCl5 at a concentration of 1.00 M?
Solution
Use the stepwise process described earlier.
Step 1.Determine the direction the reaction proceeds.
The balanced equation for the decomposition of PCl5 is
PCl5(g) ⇌ PCl3(g) + Cl2(g)
Because only the reactant is present initially Qc = 0 and the reaction will proceed to the right.
Step 2.Develop an ICE table.
| PCl5⇌ | PCl3+ | l2 | |
|---|---|---|---|
| Initial Concentration (M) | 1.00 | 0 | 0 |
| Change (M) | -x | +x | +x |
| Equilibrium Concentration (M) | 1.00 - x | x | x |
Step 3.Solve for the change and the equilibrium concentrations.
Substituting the equilibrium concentrations into the equilibrium constant equation gives
Kc = [latex]\frac{[PCl_3][CL_2]}{[PCl_5]}[/latex] = 0.0211
= [latex]\frac{(x)(x)}{(1.00 - x)}[/latex]
0.0211 = [latex]\frac{(x)(x)}{(1.00 - x)}[/latex]
0.0211 (1.00 - x) = x2
x2 + 0.0211x - 0.0211 = 0
Appendix B shows an equation of the form ax2 + bx + c = 0 can be rearranged to solve for x:
x = [latex]\frac{-b \pm \sqrt{b^{2} - 4ac}}{2a}[/latex]
In this case, a = 1, b = 0.0211, and c = −0.0211. Substituting the appropriate values for a, b, and c yields:
x = [latex]\frac{-0.0211 \pm \sqrt{(0.0211)^{2} - 4(1)(-0.0211)}}{2(1)}[/latex]
= [latex]\frac{0.0211 \pm \sqrt{(4.45 \times 10^{-4}) + (8.44 \times 10^{-2})}}{2}[/latex]
= [latex]\frac{-0.0211 \pm 0.291}{2}[/latex]
The two roots of the quadratic are, therefore,
x = [latex]\frac{-0.0211 + 0.291}{2}[/latex] = 0.135
and
x = [latex]\frac{-0.0211 - 0.291}{2}[/latex] = -0.156
For this scenario, only the positive root is physically meaningful (concentrations are either zero or positive), and so x = 0.135 M.
The equilibrium concentrations are
[PCl5] = 1.00 - 0.135 = 0.87 M
[PCl3] = x = 0.135 M
[Cl2] = x = 0.135 M
Step 4. Confirm the calculated equilibrium concentrations.
Substitution into the expression for Kc (to check the calculation) gives
Kc = [latex]\frac{[PCl_{3}][Cl_{2}]}{[PCl_{5}]} = \frac{(0.135)(0.135)}{0.87} = 0.021[/latex]
The equilibrium constant calculated from the equilibrium concentrations is equal to the value of Kc
given in the problem (when rounded to the proper number of significant figures).
Check Your Learning
Acetic acid, CH3CO2H, reacts with ethanol, C2H5OH, to form water and ethyl acetate, CH3CO2C2H5.
CH3CO2 H + C2H5OH ⇌ CH3CO2C2H5 + H2O
The equilibrium constant for this reaction with dioxane as a solvent is 4.0. What are the equilibrium concentrations for a mixture that is initially 0.15 M in CH3CO2H, 0.15 M in C2H5OH, 0.40 M in CH3CO2C2H5, and 0.40 M in H2O?
Answer: [CH3CO2H] = 0.36 M, [C2H5OH] = 0.36 M, [CH3CO2C2H5] = 0.17 M, [H2O] = 0.17 M
Check Your Learning
A 1.00-L flask is filled with 1.00 mole of H2 and 2.00 moles of I2. The value of the equilibrium constant for the reaction of hydrogen and iodine reacting to form hydrogen iodide is 50.5 under the given conditions. What are the equilibrium concentrations of H2, I2, and HI in moles/L?
H2(g) + I2(g) ⇌ 2HI(g)
Answer: [H2] = 0.06 M, [I2] = 1.06 M, [HI] = 1.88 M
Example 13.10
Calculation of Equilibrium Concentrations Using an Algebra-Simplifying Assumption
What are the concentrations at equilibrium of a 0.15 M solution of HCN?
HCN(aq) ⇌ H+(aq) + CN−(aq)Kc = 4.9 × 10−10
Solution
Using “x” to represent the concentration of each product at equilibrium gives this ICE table.
| HCN(aq)⇌ | H+(aq)+ | CN-(aq) | |
|---|---|---|---|
| Initial Concentration (M) | 0.15 | 0 | 0 |
| Change (M) | -x | +x | +x |
| Equilibrium Concentration (M) | 0.15 - x | x | x |
Substitute the equilibrium concentration terms into the Kc expression
Kc = [latex]\frac{(x)(x)}{0.15 - x}[/latex]
rearrange to the quadratic form and solve for x
x2 + 4.9 × 10−10 − 7.35 × 10−11 = 0
x = 8.56 × 10−6 M (3 sig. figs.) = 8.6 × 10−6 M (2 sig. figs.)
Thus [H+] = [CN–] = x = 8.6 × 10–6 M and [HCN] = 0.15 – x = 0.15 M.
Note in this case that the change in concentration is significantly less than the initial concentration (a consequence of the small K), and so the initial concentration experiences a negligible change:
if x ≪ 0.15 M, then (0.15 – x) ≈ 0.15
This approximation allows for a more expedient mathematical approach to the calculation that avoids the need to solve for the roots of a quadratic equation:
Kc = [latex]\frac{(x)(x)}{0.15 - x}[/latex] ≈ [latex]\frac{x^2}{0.15}[/latex]
4.9 x 10-10 = [latex]\frac{x^2}{0.15}[/latex]
x2 = (0.15)(4.9 × 10−10) = 7.4 × 10−11
x = [latex]\sqrt{7.4 \times 10^{-11}} = 8.6 \times 10^{-6} M[/latex]
The value of x calculated is, indeed, much less than the initial concentration
8.6 × 10−6 ≪ 0.15
and so the approximation was justified. If this simplified approach were to yield a value for x that did not justify the approximation, the calculation would need to be repeated without making the approximation.
Check Your Learning
What are the equilibrium concentrations in a 0.25 M NH3 solution?
NH3(aq) + H2O(l) ⇌ NH4+(aq) + OH−(aq) Kc = 1.8 × 10−5
Answer: [OH−] = [NH4+] = 0.0021 M; [NH3] = 0.25 M
Key Terms
equilibrium state of a reversible reaction in which the forward and reverse processes occur at equal rates
equilibrium constant (K) value of the reaction quotient for a system at equilibrium; may be expressed using concentrations (Kc) or partial pressures (Kp)
heterogeneous equilibria equilibria in which reactants and products occupy two or more different phases
homogeneous equilibria equilibria in which all reactants and products occupy the same phase
law of mass action when a reversible reaction has attained equilibrium at a given temperature, the reaction quotient remains constant
Le Châtelier’s principle an equilibrium subjected to stress will shift in a way to counter the stress and re-establish equilibrium
reaction quotient (Q) mathematical function describing the relative amounts of reactants and products in a reaction mixture; may be expressed in terms of concentrations (Qc) or pressures (Qp)
reversible reaction chemical reaction that can proceed in both the forward and reverse directions under given conditions
Key Equations
- Qc = [latex]\frac{[C]^{x}[D]^{y}}{[A]^{m}[B]^{n}}[/latex] for the reaction mA + nB ⇌ xC + yD
- Qp = [latex]\frac{(P_C)^x(P_D)^y}{(P_A)^m(P_B)^n}[/latex] for the reaction mA + nB ⇌ xC + y
- P = MRT
- Kc = Qc at equilibrium
- Kp = Qp at equilibrium
- KP = Kc (RT)Δn
Summary
Chemical Equilibria
A reversible reaction is at equilibrium when the forward and reverse processes occur at equal rates. Chemical equilibria are dynamic processes characterized by constant amounts of reactant and product species.
Equilibrium Constants
The composition of a reaction mixture may be represented by a mathematical function known as the reaction quotient,
Q. For a reaction at equilibrium, the composition is constant, and Q is called the equilibrium constant, K.
A homogeneous equilibrium is an equilibrium in which all components are in the same phase. A heterogeneous equilibrium is an equilibrium in which components are in two or more phases.
Shifting Equilibria: Le Châtelier’s Principle
Systems at equilibrium can be disturbed by changes to temperature, concentration, and, in some cases, volume and pressure. The system’s response to these disturbances is described by Le Châtelier’s principle: An equilibrium system subjected to a disturbance will shift in a way that counters the disturbance and re-establishes equilibrium. A catalyst will increase the rate of both the forward and reverse reactions of a reversible process, increasing the rate at which
equilibrium is reached but not altering the equilibrium mixture’s composition (K does not change).
Equilibrium Calculations
Calculating values for equilibrium constants and/or equilibrium concentrations is of practical benefit to many applications. A mathematical strategy that uses initial concentrations, changes in concentrations, and equilibrium concentrations (and goes by the acronym ICE) is useful for several types of equilibrium calculations.
Exercises
-
-
- What does it mean to describe a reaction as “reversible”?
- When writing an equation, how is a reversible reaction distinguished from a nonreversible reaction?
- If a reaction is reversible, when can it be said to have reached equilibrium?
- Is a system at equilibrium if the rate constants of the forward and reverse reactions are equal?
- If the concentrations of products and reactants are equal, is the system at equilibrium?
- Explain why there may be an infinite number of values for the reaction quotient of a reaction at a given temperature but there can be only one value for the equilibrium constant at that temperature.
- Explain why an equilibrium between Br2(l) and Br2(g) would not be established if the container were not a closed vessel shown in Figure13.4.
- If you observe the following reaction at equilibrium, is it possible to tell whether the reaction started with pure NO2 or with pure N2O4?
2NO2(g) ⇌ N2O4(g) - Among the solubility rules previously discussed is the statement: All chlorides are soluble except Hg2Cl2, AgCl, PbCl2, and CuCl.
- Write the expression for the equilibrium constant for the reaction represented by the equation
AgCl(s) ⇌ Ag+(aq) + Cl−(aq). Is Kc > 1, < 1, or ≈ 1? Explain your answer.
- Write the expression for the equilibrium constant for the reaction represented by the equation
Pb2+(aq) + 2Cl−(aq) ⇌ PbCl2(s). Is Kc > 1, < 1, or ≈ 1? Explain your answer.
- Write the expression for the equilibrium constant for the reaction represented by the equation
- Among the solubility rules previously discussed is the statement: Carbonates, phosphates, borates, and arsenates—except those of the ammonium ion and the alkali metals—are insoluble.
- Write the expression for the equilibrium constant for the reaction represented by the equation
CaCO3(s) ⇌ Ca2+(aq) + CO3 2−(aq). Is Kc > 1, < 1, or ≈ 1? Explain your answer.
- Write the expression for the equilibrium constant for the reaction represented by the equation
2BA2+(aq) + 2PO4 3-(aq) ⇌ Ba3(PO4)2(s). Is Kc > 1, < 1, or ≈ 1? Explain your answer.
- Write the expression for the equilibrium constant for the reaction represented by the equation
- Benzene is one of the compounds used as octane enhancers in unleaded gasoline. It is manufactured by the catalytic conversion of acetylene to benzene: 3C2H2(g) ⇌ C6H6(g). Which value of Kc would make this reaction most useful commercially? Kc ≈ 0.01, Kc ≈ 1, or Kc ≈ 10. Explain your answer.
- Show that the complete chemical equation, the total ionic equation, and the net ionic equation for the reaction represented by the equation KI(aq) + I2(aq) ⇌ KI3(aq) give the same expression for the reaction quotient. KI3 is composed of the ions K+ and I3 −.
- For a titration to be effective, the reaction must be rapid and the yield of the reaction must essentially be 100%. Is Kc > 1, < 1, or ≈ 1 for a titration reaction?
- For a precipitation reaction to be useful in a gravimetric analysis, the product of the reaction must be insoluble. Is Kc > 1, < 1, or ≈ 1 for a useful precipitation reaction?
- Write the mathematical expression for the reaction quotient, Qc, for each of the following reactions:
- CH4(g) + Cl2(g) ⇌ CH3 Cl(g) + HCl(g)
- N2(g) + O2(g) ⇌ 2NO(g)
- 2SO2(g) + O2(g) ⇌ 2SO3(g)
- BaSO3(s) ⇌ BaO(s) + SO2(g)
- P4(g) + 5O2(g) ⇌ P4O10(s)
- Br2(g) ⇌ 2Br(g)
- CH4(g) + 2O2(g) ⇌ CO2(g) + 2H2O(l)
- CuSO4 ·5H2O(s) ⇌ CuSO4(s) + 5H2O(g)
- Write the mathematical expression for the reaction quotient, Qc, for each of the following reactions:
- N2(g) + 3H2(g) ⇌ 2NH3(g)
- 4NH3(g) + 5O2(g) ⇌ 4NO(g) + 6H2O(g)
- N2O4(g) ⇌ 2NO2(g)
- CO2(g) + H2(g) ⇌ CO(g) + H2O(g)
- NH4 Cl(s) ⇌ NH3(g) + HCl(g)
- 2Pb(NO3)2(s) ⇌ 2PbO(s) + 4NO (g) + O (g)
- 2H2(g) + O2(g) ⇌ 2H2O(l)
- S8(g) ⇌ 8S(g)
- The initial concentrations or pressures of reactants and products are given for each of the following systems. Calculate the reaction quotient and determine the direction in which each system will proceed to reach equilibrium.
- 2NH3(g) ⇌ N2(g) + 3H2(g)Kc = 17; [NH3] = 0.20 M, [N2] = 1.00 M, [H2] = 1.00 M
- 2NH3(g) ⇌ N2(g) + 3H2(g)KP = 6.8 × 104; NH3 = 3.0 atm, N2 = 2.0 atm, H2 = 1.0 atm
- 2SO3(g) ⇌ 2SO2(g) + O2 (g)Kc = 0.230; [SO3] = 0.00 M, [SO2] = 1.00 M, [O2] = 1.00 M
- 2SO3(g) ⇌ 2SO2(g) + O2(g)KP = 16.5; SO3 = 1.00 atm, SO2 = 1.00 atm, O2 = 1.00 atm
- 2NO(g) + Cl2(g) ⇌ 2NOCl(g)Kc = 4.6 × 104; [NO] = 1.00 M, [Cl2] = 1.00 M, [NOCl] = 0 M
- N2(g) + O2(g) ⇌ 2NO(g)KP = 0.050; NO = 10.0 atm, N2 = O2 = 5 atm
- The initial concentrations or pressures of reactants and products are given for each of the following systems. Calculate the reaction quotient and determine the direction in which each system will proceed to reach equilibrium.
- 2NH3(g) ⇌ N2(g) + 3H2(g)Kc = 17; [NH3] = 0.50 M, [N2] = 0.15 M, [H2] = 0.12 M
- 2NH3(g) ⇌ N2(g) + 3H2(g)KP = 6.8 × 104; NH3 = 2.00 atm, N2 = 10.00 atm, H2 = 10.00 atm
- 2SO3(g) ⇌ 2SO2(g) + O2(g)Kc = 0.230; [SO3] = 2.00 M, [SO2] = 2.00 M, [O2] = 2.00 M
- 2SO3(g) ⇌ 2SO2(g) + O2(g)KP = 6.5 atm; SO2 = 1.00 atm, O2 = 1.130 atm, SO3 = 0 atm
- 2NO(g) + Cl2(g) ⇌ 2NOCl(g)KP = 2.5 × 103; NO = 1.00 atm, Cl2 = 1.00 atm, NOCl = 0 atm
- N2(g) + O2(g) ⇌ 2NO(g)Kc = 0.050; [N2] = 0.100 M, [O2] = 0.200 M, [NO] = 1.00 M
- The following reaction has KP = 4.50 × 10−5 at 720 K.
N2(g) + 3H2(g) ⇌ 2NH3(g)
If a reaction vessel is filled with each gas to the partial pressures listed, in which direction will it shift to reach equilibrium? P(NH3) = 93 atm, P(N2) = 48 atm, and P(H2) = 52 atm
- Determine if the following system is at equilibrium. If not, in which direction will the system need to shift to reach equilibrium?
SO2 Cl2(g) ⇌ SO2(g) + Cl2(g)
[SO2Cl2] = 0.12 M, [Cl2] = 0.16 M and [SO2] = 0.050 M. Kc for the reaction is 0.078.
- Which of the systems described in Exercise13.15are homogeneous equilibria? Which are heterogeneous equilibria?
- Which of the systems described in Exercise13.16are homogeneous equilibria? Which are heterogeneous equilibria?
- For which of the reactions in Exercise 13.15 does Kc (calculated using concentrations) equal KP (calculated using pressures)?
- For which of the reactions in Exercise 13.16 does Kc (calculated using concentrations) equal KP (calculated using pressures)?
- Convert the values of Kc to values of KP or the values of KP to values of Kc.
- N2(g) + 3H2(g) ⇌ 2NH3(g)Kc = 0.50 at 400 °C
- H2(g) + I2(g) ⇌ 2HI(g)Kc = 50.2 at 448 °C
- Na2 SO4 ·10H2 O(s) ⇌ Na2 SO4(s) + 10H2 O(g)KP = 4.08 × 10−25 at 25 °C
- H2 O(l) ⇌ H2 O(g)KP = 0.122 at 50 °C
- Convert the values of Kc to values of KP or the values of KP to values of Kc.
- Cl2(g) + Br2(g) ⇌ 2BrCl(g)Kc = 4.7 × 10−2 at 25 °C
- 2SO2(g) + O2(g) ⇌ 2SO3(g)KP = 48.2 at 500 °C
- CaCl2 ·6H2 O(s) ⇌ CaCl2(s) + 6H2 O(g)KP = 5.09 × 10−44 at 25 °C
- H2 O(l) ⇌ H2 O(g)KP = 0.196 at 60 °C
- What is the value of the equilibrium constant expression for the change H2 O(l) ⇌ H2 O(g) at 30 °C?
- Write the expression of the reaction quotient for the ionization of HOCN in water
- Write the reaction quotient expression for the ionization of NH3 in water.
- What is the approximate value of the equilibrium constant KP for the change
C2 H5 OC2 H5(l) ⇌ C2 H5 OC2 H5(g) at 25 °C. (The equilibrium vapor pressure for this substance is 570 torr at 25°C.)
- The following equation represents a reversible decomposition:
[latex]\text{CaCO}_3(s)\;{\rightleftharpoons}\;\text{CaO}(s)\;+\;\text{CO}_2(g)[/latex]Under what conditions will decomposition in a closed container proceed to completion so that no CaCO3 remains?
- Explain how to recognize the conditions under which changes in pressure would affect systems at equilibrium.
- What property of a reaction can we use to predict the effect of a change in temperature on the value of an equilibrium constant?
- What would happen to the color of the solution in part (b) of Figure 1 if a small amount of NaOH were added and Fe(OH)3 precipitated? Explain your answer.
- The following reaction occurs when a burner on a gas stove is lit:
[latex]\text{CH}_4(g)\;+\;2\text{O}_2(g)\;{\rightleftharpoons}\;\text{CO}_2(g)\;+\;2\text{H}_2\text{O}(g)[/latex]Is an equilibrium among CH4, O2, CO2, and H2O established under these conditions? Explain your answer.
- A necessary step in the manufacture of sulfuric acid is the formation of sulfur trioxide, SO3, from sulfur dioxide, SO2, and oxygen, O2, shown here. At high temperatures, the rate of formation of SO3 is higher, but the equilibrium amount (concentration or partial pressure) of SO3 is lower than it would be at lower temperatures.
[latex]2\text{SO}_2(g)\;+\;\text{O}_2(g)\;{\longrightarrow}\;2\text{SO}_3(g)[/latex]- Does the equilibrium constant for the reaction increase, decrease, or remain about the same as the temperature increases?
- Is the reaction endothermic or exothermic?
- Suggest four ways in which the concentration of hydrazine, N2H4, could be increased in an equilibrium described by the following equation:
[latex]\text{N}_2(g)\;+\;2\text{H}_2(g)\;{\rightleftharpoons}\;\text{N}_2\text{H}_4(g)\;\;\;\;\;\;\;{\Delta}H = 95\;\text{kJ}[/latex] - Suggest four ways in which the concentration of PH3 could be increased in an equilibrium described by the following equation:
[latex]\text{P}_4(g)\;+\;6\text{H}_2(g)\;{\rightleftharpoons}\;4\text{PH}_3(g)\;\;\;\;\;\;\;{\Delta}H = 110.5\;\text{kJ}[/latex] - How will an increase in temperature affect each of the following equilibria? How will a decrease in the volume of the reaction vessel affect each?
(a) [latex]2\text{NH}_3(g)\;{\rightleftharpoons}\;\text{N}_2(g)\;+\;3\text{H}_2(g)\;\;\;\;\;\;\;{\Delta}H = 92\;\text{kJ}[/latex]
(b) [latex]\text{N}_2(g)\;+\;\text{O}_2(g)\;{\rightleftharpoons}\;2\text{NO}(g)\;\;\;\;\;\;\;{\Delta}H = 181\;\text{kJ}[/latex]
(c) [latex]2\text{O}_3(g)\;{\rightleftharpoons}\;3\text{O}_2(g)\;\;\;\;\;\;\;{\Delta}H = -285\;\text{kJ}[/latex]
(d) [latex]\text{CaO}(s)\;+\;\text{CO}_2(g)\;{\rightleftharpoons}\;\text{CaCO}_3(s)\;\;\;\;\;\;\;{\Delta}H = -176\;\text{kJ}[/latex]
- How will an increase in temperature affect each of the following equilibria? How will a decrease in the volume of the reaction vessel affect each?
- [latex]2\text{H}_2\text{O}(g)\;{\rightleftharpoons}\;2\text{H}_2(g)\;+\;\text{O}_2(g)\;\;\;\;\;\;\;{\Delta}H = 484\;\text{kJ}[/latex]
- [latex]\text{N}_2(g)\;+\;3\text{H}_2(g)\;{\rightleftharpoons}\;2\text{NH}_3(g)\;{\Delta}H = -92.2\;\text{kJ}[/latex]
- [latex]2\text{Br}(g)\;{\rightleftharpoons}\;\text{Br}_2(g)\;\;\;\;\;\;\;{\Delta}H = -224\;\text{kJ}[/latex]
- [latex]\text{H}_2(g)\;+\;\text{I}_2(s)\;{\rightleftharpoons}\;2\text{HI}(g)\;\;\;\;\;\;\;{\Delta}H = 53\;\text{kJ}[/latex]
- Water gas is a 1:1 mixture of carbon monoxide and hydrogen gas and is called water gas because it is formed from steam and hot carbon in the following reaction: [latex]\text{H}_2\text{O}(g)\;+\;\text{C}(s)\;{\rightleftharpoons}\;\text{H}_2(g)\;+\;\text{CO}(g)[/latex]. Methanol, a liquid fuel that could possibly replace gasoline, can be prepared from water gas and hydrogen at high temperature and pressure in the presence of a suitable catalyst.
(a) Write the expression for the equilibrium constant (Kc) for the reversible reaction
[latex]2\text{H}_2(g)\;+\;\text{CO}(g)\;{\rightleftharpoons}\;\text{CH}_3\text{OH}(g)\;\;\;\;\;\;\;{\Delta}H = -90.2\;\text{kJ}[/latex]
(b) What will happen to the concentrations of H2, CO, and CH3OH at equilibrium if more H2 is added?
(c) What will happen to the concentrations of H2, CO, and CH3OH at equilibrium if CO is removed?
(d) What will happen to the concentrations of H2, CO, and CH3OH at equilibrium if CH3OH is added?
(e) What will happen to the concentrations of H2, CO, and CH3OH at equilibrium if the temperature of the system is increased?
(f) What will happen to the concentrations of H2, CO, and CH3OH at equilibrium if more catalyst is added?
- Nitrogen and oxygen react at high temperatures.
(a) Write the expression for the equilibrium constant (Kc) for the reversible reaction
[latex]\text{N}_2(g)\;+\;\text{O}_2(g)\;{\rightleftharpoons}\;2\text{NO}(g)\;\;\;\;\;\;\;{\Delta}H = 181\;\text{kJ}[/latex]
(b) What will happen to the concentrations of N2, O2, and NO at equilibrium if more O2 is added?
(c) What will happen to the concentrations of N2, O2, and NO at equilibrium if N2 is removed?
(d) What will happen to the concentrations of N2, O2, and NO at equilibrium if NO is added?
(e) What will happen to the concentrations of N2, O2, and NO at equilibrium if the pressure on the system is increased by reducing the volume of the reaction vessel?
(f) What will happen to the concentrations of N2, O2, and NO at equilibrium if the temperature of the system is increased?
(g) What will happen to the concentrations of N2, O2, and NO at equilibrium if a catalyst is added?
- Water gas, a mixture of H2 and CO, is an important industrial fuel produced by the reaction of steam with red hot coke, essentially pure carbon.
(a) Write the expression for the equilibrium constant for the reversible reaction
[latex]\text{C}(s)\;+\;\text{H}_2\text{O}(g)\;{\rightleftharpoons}\;\text{CO}(g)\;+\;\text{H}_2(g)\;\;\;\;\;\;\;{\Delta}H = 131.30\;\text{kJ}[/latex]
(b) What will happen to the concentration of each reactant and product at equilibrium if more C is added?
(c) What will happen to the concentration of each reactant and product at equilibrium if H2O is removed?
(d) What will happen to the concentration of each reactant and product at equilibrium if CO is added?
(e) What will happen to the concentration of each reactant and product at equilibrium if the temperature of the system is increased?
- Pure iron metal can be produced by the reduction of iron(III) oxide with hydrogen gas.
(a) Write the expression for the equilibrium constant (Kc) for the reversible reaction
[latex]\text{Fe}_2\text{O}_3(s)\;+\;3\text{H}_2(g)\;{\rightleftharpoons}\;2\text{Fe}(s)\;+\;3\text{H}_2\text{O}(g)\;\;\;\;\;\;\;{\Delta}H = 98.7\;\text{kJ}[/latex]
(b) What will happen to the concentration of each reactant and product at equilibrium if more Fe is added?
(c) What will happen to the concentration of each reactant and product at equilibrium if H2O is removed?
(d) What will happen to the concentration of each reactant and product at equilibrium if H2 is added?
(e) What will happen to the concentration of each reactant and product at equilibrium if the pressure on the system is increased by reducing the volume of the reaction vessel?
(f) What will happen to the concentration of each reactant and product at equilibrium if the temperature of the system is increased?
- Ammonia is a weak base that reacts with water according to this equation:
[latex]\text{NH}_3(aq)\;+\;\text{H}_2\text{O}(l)\;{\rightleftharpoons}\;\text{NH}_4^{\;\;+}(aq)\;+\;\text{OH}^{-}(aq)[/latex]Will any of the following increase the percent of ammonia that is converted to the ammonium ion in water?
(a) Addition of NaOH
(b) Addition of HCl
(c) Addition of NH4Cl
- Acetic acid is a weak acid that reacts with water according to this equation:
[latex]\text{CH}_3\text{CO}_2\text{H}(aq)\;+\;\text{H}_2\text{O}(aq)\;{\rightleftharpoons}\;\text{H}_3\text{O}^{+}(aq)\;+\;\text{CH}_3\text{CO}_2^{\;\;-}(aq)[/latex]Will any of the following increase the percent of acetic acid that reacts and produces [latex]\text{CH}_3\text{CO}_2^{\;\;-}[/latex] ion?
(a) Addition of HCl
(b) Addition of NaOH
(c) Addition of NaCH3CO2
- Suggest two ways in which the equilibrium concentration of Ag+ can be reduced in a solution of Na+, Cl−, Ag+, and [latex]\text{NO}_3^{\;\;-}[/latex], in contact with solid AgCl.
[latex]\text{Na}^{+}(aq)\;+\;\text{Cl}^{-}(aq)\;+\;\text{Ag}^{+}(aq)\;+\;\text{NO}_3^{\;\;-}(aq)\;{\rightleftharpoons}\;\text{AgCl}(s)\;+\;\text{Na}^{+}(aq)\;+\;\text{NO}_3^{\;\;-}(aq)[/latex]
[latex]{\Delta}H = -65.9\;\text{kJ}[/latex] - How can the pressure of water vapor be increased in the following equilibrium?
[latex]\text{H}_2\text{O}(l)\;{\rightleftharpoons}\;\text{H}_2\text{O}(g)\;\;\;\;\;\;\;{\Delta}H = 41\;\text{kJ}[/latex] - Additional solid silver sulfate, a slightly soluble solid, is added to a solution of silver ion and sulfate ion at equilibrium with solid silver sulfate.
[latex]2\text{Ag}^{+}(aq)\;+\;\text{SO}_4^{\;\;2-}(aq)\;{\rightleftharpoons}\;\text{Ag}_2\text{SO}_4(s)[/latex]Which of the following will occur?
(a) Ag+ or [latex]\text{SO}_4^{\;\;2-}[/latex] concentrations will not change.
(b) The added silver sulfate will dissolve.
(c) Additional silver sulfate will form and precipitate from solution as Ag+ ions and [latex]\text{SO}_4^{\;\;2-}[/latex] ions combine.
(d) The Ag+ ion concentration will increase and the [latex]\text{SO}_4^{\;\;2-}[/latex] ion concentration will decrease.
- The amino acid alanine has two isomers, α-alanine and β-alanine. When equal masses of these two compounds are dissolved in equal amounts of a solvent, the solution of α-alanine freezes at the lowest temperature. Which form, α-alanine or β-alanine, has the larger equilibrium constant for ionization [latex](\text{HX}\;{\rightleftharpoons}\;\text{H}^{+}\;+\;\text{X}^{-})[/latex]?
- A reaction is represented by this equation:
A(aq) + 2B(aq) ⇌ 2C(aq)Kc = 1 × 103- Write the mathematical expression for the equilibrium constant.
- Using concentrations ≤1 M, identify two sets of concentrations that describe a mixture of A, B, and C at equilibrium.
- A reaction is represented by this equation:
2W(aq) ⇌ X(aq) + 2Y(aq)Kc = 5 × 10−4- Write the mathematical expression for the equilibrium constant.
- Using concentrations of ≤1 M, identify two sets of concentrations that describe a mixture of W, X, and Y at equilibrium.
- What is the value of the equilibrium constant at 500 °C for the formation of NH3 according to the following equation?
N2(g) + 3H2(g) ⇌ 2NH3(g)
An equilibrium mixture of NH3(g), H2(g), and N2(g) at 500 °C was found to contain 1.35 M H2, 1.15 M N2, and 4.12 × 10−1 M NH3.
- Hydrogen is prepared commercially by the reaction of methane and water vapor at elevated temperatures.
CH4(g) + H2 O(g) ⇌ 3H2(g) + CO(g)
What is the equilibrium constant for the reaction if a mixture at equilibrium contains gases with the following concentrations: CH4, 0.126 M; H2O, 0.242 M; CO, 0.126 M; H2 1.15 M, at a temperature of 760 °C?
- A 0.72-mol sample of PCl5 is put into a 1.00-L vessel and heated. At equilibrium, the vessel contains 0.40 mol of PCl3(g) and 0.40 mol of Cl2(g). Calculate the value of the equilibrium constant for the decomposition of PCl5 to PCl3 and Cl2 at this temperature.
- At 1 atm and 25 °C, NO2 with an initial concentration of 1.00 M is 0.0033% decomposed into NO and O2. Calculate the value of the equilibrium constant for the reaction.
2NO2(g) ⇌ 2NO(g) + O2(g)
- Calculate the value of the equilibrium constant KP for the reaction 2NO(g) + Cl2(g) ⇌ 2NOCl(g) from these equilibrium pressures: NO, 0.050 atm; Cl2, 0.30 atm; NOCl, 1.2 atm.
- When heated, iodine vapor dissociates according to this equation:
I2(g) ⇌ 2I(g)
At 1274 K, a sample exhibits a partial pressure of I2 of 0.1122 atm and a partial pressure due to I atoms of 0.1378 atm. Determine the value of the equilibrium constant, KP, for the decomposition at 1274 K.
- A sample of ammonium chloride was heated in a closed container.
NH4 Cl(s) ⇌ NH3(g) + HCl(g)
At equilibrium, the pressure of NH3(g) was found to be 1.75 atm. What is the value of the equilibrium constant KP
for the decomposition at this temperature?
- At a temperature of 60 °C, the vapor pressure of water is 0.196 atm. What is the value of the equilibrium constant KP for the vaporization equilibrium at 60 °C?
H2 O(l) ⇌ H2 O(g)
- Complete the changes in concentrations (or pressure, if requested) for each of the following reactions.
2SO3(g) ⇌ 2SO2(g) + O2(g)
___ ___ +x
___ ___ 0.125M
4NH3(g) + 3O2(g) ⇌ 2N2(g) + 6H2 O(g)
___ 3x ___ ___
___ 0.24 M ___ ___
Change in pressure:
2CH4(g) ⇌ C2 H2(g) + 3H2(g)
___ x ___
___ 25 torr ___
Change in pressure:
CH4(g) + H2 O(g) ⇌ CO(g) + 3H2(g)
___ x ___ ___
___ 5 atm ___ ___
NH4 Cl(s) ⇌ NH3(g) +HCl(g)
x ___
1.03 × 10−4 M ___
change in pressure:
Ni(s) + 4CO(g)⇌ Ni(CO)4(g)
4x ___
0.40 atm ___
- Complete the changes in concentrations (or pressure, if requested) for each of the following reactions.
2H2(g) + O2(g) ⇌ 2H2 O(g)
___ ___ +2x
___ ___ 1.50M
CS2(g) + 4H2(g) ⇌ CH4(g) + 2H2S(g)
x ___ ___ ___
0.020 M ___ ___ ___
Change in pressure:
H2(g) + Cl2(g) ⇌ 2HCl(g)
x ___ ___
1.50 atm ___ ___
Change in pressure:
2NH3(g) + 2O2(g) ⇌ N2 O(g) + 3H2 O(g)
___ ___ ___ x
___ ___ ___ 60.6 torr
NH4 HS(s) ⇌ NH3(g) +H2 S(g)
x ___
9.8 × 10−6M ___
Change in pressure:
Fe(s) + 5CO(g) ⇌ Fe(CO)5(g)
___ x
___ 0.012 atm
- Why are there no changes specified for Ni in Exercise 13.60, part (f)? What property of Ni does change?
- Why are there no changes specified for NH4HS in Exercise 13.61, part (e)? What property of NH4HS does change?
- Analysis of the gases in a sealed reaction vessel containing NH3, N2, and H2 at equilibrium at 400 °C established the concentration of N2 to be 1.2 M and the concentration of H2 to be 0.24 M.
N2(g) + 3H2(g) ⇌ 2NH3(g)Kc = 0.50 at 400 °C
Calculate the equilibrium molar concentration of NH3.
- Calculate the number of moles of HI that are at equilibrium with 1.25 mol of H2 and 1.25 mol of I2 in a 5.00−L flask at 448 °C.
H2 + I2 ⇌ 2HIKc = 50.2 at 448 °C
- What is the pressure of BrCl in an equilibrium mixture of Cl2, Br2, and BrCl if the pressure of Cl2 in the mixture is 0.115 atm and the pressure of Br2 in the mixture is 0.450 atm?
Cl2(g) + Br2(g) ⇌ 2BrCl(g)KP = 4.7 × 10−2
- What is the pressure of CO2 in a mixture at equilibrium that contains 0.50 atm H2, 2.0 atm of H2O, and 1.0 atm of CO at 990 °C?
H2(g) + CO2(g) ⇌ H2 O(g) + CO(g)KP = 1.6 at 990 °C
- Cobalt metal can be prepared by reducing cobalt(II) oxide with carbon monoxide.
CoO(s) + CO(g) ⇌ Co(s) + CO2(g)Kc = 4.90 × 102 at 550 °C
What concentration of CO remains in an equilibrium mixture with [CO2] = 0.100 M?
- Carbon reacts with water vapor at elevated temperatures.
C(s) + H2 O(g) ⇌ CO(g) + H2(g)Kc = 0.2 at 1000 °C
Assuming a reaction mixture initially contains only reactants, what is the concentration of CO in an equilibrium mixture with [H2O] = 0.500 M at 1000 °C?
- Sodium sulfate 10−hydrate, Na2SO4·10H2O, dehydrates according to the equation
Na2 SO4 ·10H2 O(s) ⇌ Na2 SO4(s) + 10H2 O(g)KP = 4.08 × 10−25 at 25 °C
What is the pressure of water vapor at equilibrium with a mixture of Na2SO4·10H2O and NaSO4?
- Calcium chloride 6−hydrate, CaCl2·6H2O, dehydrates according to the equation
CaCl2 ·6H2 O(s) ⇌ CaCl2(s) + 6H2 O(g)KP = 5.09 × 10−44 at 25 °C
What is the pressure of water vapor at equilibrium with a mixture of CaCl2·6H2O and CaCl2 at 25 °C?
- A student solved the following problem and found the equilibrium concentrations to be [SO2] = 0.590 M, [O2]
= 0.0450 M, and [SO3] = 0.260 M. How could this student check the work without reworking the problem? The problem was: For the following reaction at 600 °C:
2SO2(g) + O2(g) ⇌ 2SO3(g)Kc = 4.32
- A student solved the following problem and found [N2O4] = 0.16 M at equilibrium. How could this student recognize that the answer was wrong without reworking the problem? The problem was: What is the equilibrium concentration of N2O4 in a mixture formed from a sample of NO2 with a concentration of 0.10 M?
2NO2(g) ⇌ N2 O4(g)Kc = 160
- Assume that the change in concentration of N2O4 is small enough to be neglected in the following problem.
Calculate the equilibrium concentration of both species in 1.00 L of a solution prepared from 0.129 mol of N2O4 with chloroform as the solvent.N2 O4(g) ⇌ 2NO2(g)Kc = 1.07 × 10−5 in chloroform
Confirm that the change is small enough to be neglected.
- Assume that the change in concentration of COCl2 is small enough to be neglected in the following problem.
Calculate the equilibrium concentration of all species in an equilibrium mixture that results from the decomposition of COCl2 with an initial concentration of 0.3166 M.COCl2(g) ⇌ CO(g) + Cl2(g)Kc = 2.2 × 10−10
Confirm that the change is small enough to be neglected.
- Assume that the change in pressure of H2S is small enough to be neglected in the following problem.
Calculate the equilibrium pressures of all species in an equilibrium mixture that results from the decomposition of H2S with an initial pressure of 0.824 atm.2H2 S(g) ⇌ 2H2(g) + S2(g)KP = 2.2 × 10−6
Confirm that the change is small enough to be neglected.
- What are all concentrations after a mixture that contains [H2O] = 1.00 M and [Cl2O] = 1.00 M comes to equilibrium at 25 °C?
H2 O(g) + Cl2 O(g) ⇌ 2HOCl(g)Kc = 0.0900
- What are the concentrations of PCl5, PCl3, and Cl2 in an equilibrium mixture produced by the decomposition of a sample of pure PCl5 with [PCl5] = 2.00 M?
PCl5(g) ⇌ PCl3(g) + Cl2(g)Kc = 0.0211
- Calculate the number of grams of HI that are at equilibrium with 1.25 mol of H2 and 63.5 g of iodine at 448°C.
H2 + I2 ⇌ 2HIKc = 50.2 at 448 °C
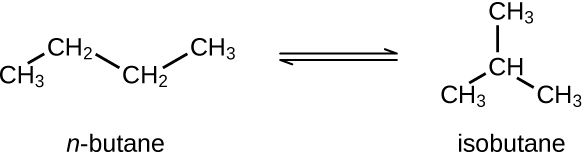 Butane exists as two isomers, n−butane and isobutane.
Butane exists as two isomers, n−butane and isobutane.
KP = 2.5 at 25 °C
What is the pressure of isobutane in a container of the two isomers at equilibrium with a total pressure of 1.22 atm?
- What is the minimum mass of CaCO3 required to establish equilibrium at a certain temperature in a 6.50-L container if the equilibrium constant (Kc) is 0.50 for the decomposition reaction of CaCO3 at that temperature?
CaCO3(s) ⇌ CaO(s) + CO2(g)
- The equilibrium constant (Kc) for this reaction is 1.60 at 990 °C:
H2(g) + CO2(g) ⇌ H2 O(g) + CO(g)
Calculate the number of moles of each component in the final equilibrium mixture obtained from adding 1.00 mol of H2, 2.00 mol of CO2, 0.750 mol of H2O, and 1.00 mol of CO to a 5.00-L container at 990 °C.
- In a 3.0-L vessel, the following equilibrium partial pressures are measured: N2, 190 torr; H2, 317 torr; NH3,
1.00 × 103 torr.
N2(g) + 3H2(g) ⇌ 2NH3(g)
How will the partial pressures of H2, N2, and NH3 change if H2 is removed from the system? Will they increase, decrease, or remain the same?
Hydrogen is removed from the vessel until the partial pressure of nitrogen, at equilibrium, is 250 torr. Calculate the partial pressures of the other substances under the new conditions. - The equilibrium constant (Kc) for this reaction is 5.0 at a given temperature.
CO(g) + H2 O(g) ⇌ CO2(g) + H2(g)
On analysis, an equilibrium mixture of the substances present at the given temperature was found to contain 0.20 mol of CO, 0.30 mol of water vapor, and 0.90 mol of H2 in a liter. How many moles of CO2 were there in the equilibrium mixture?
Maintaining the same temperature, additional H2 was added to the system, and some water vapor was removed by drying. A new equilibrium mixture was thereby established containing 0.40 mol of CO, 0.30 mol of water vapor, and 1.2 mol of H2 in a liter. How many moles of CO2 were in the new equilibrium mixture? Compare this with the quantity in part (a), and discuss whether the second value is reasonable. Explain how it is possible for the water vapor concentration to be the same in the two equilibrium solutions even though some vapor was removed before the second equilibrium was established. - Antimony pentachloride decomposes according to this equation:
SbCl5(g) ⇌ SbCl3(g) + Cl2(g)
An equilibrium mixture in a 5.00-L flask at 448 °C contains 3.85 g of SbCl5, 9.14 g of SbCl3, and 2.84 g of Cl2. How many grams of each will be found if the mixture is transferred into a 2.00-L flask at the same temperature?
- Consider the equilibrium
4NO2(g) + 6H2 O(g) ⇌ 4NH3(g) + 7O2(g)
- What is the expression for the equilibrium constant (Kc) of the reaction?
- How must the concentration of NH3 change to reach equilibrium if the reaction quotient is less than the equilibrium constant?
- If the reaction were at equilibrium, how would an increase in the volume of the reaction vessel affect the pressure of NO2?
- If the change in the pressure of NO2 is 28 torr as a mixture of the four gases reaches equilibrium, how much will the pressure of O2 change?
- The binding of oxygen by hemoglobin (Hb), giving oxyhemoglobin (HbO2), is partially regulated by the concentration of H3O+ and dissolved CO2 in the blood. Although the equilibrium is complicated, it can be summarized as
HbO2(aq) + H3 O+(aq) + CO2(g) ⇌ CO2 −Hb−H+ + O2(g) + H2 O(l)
Write the equilibrium constant expression for this reaction.
Explain why the production of lactic acid and CO2 in a muscle during exertion stimulates release of O2 from the oxyhemoglobin in the blood passing through the muscle. - Liquid N2O3 is dark blue at low temperatures, but the color fades and becomes greenish at higher temperatures as the compound decomposes to NO and NO2. At 25 °C, a value of KP = 1.91 has been established for this decomposition. If 0.236 moles of N2O3 are placed in a 1.52-L vessel at 25 °C, calculate the equilibrium partial pressures of N2O3(g), NO2(g), and NO(g).
- A 1.00-L vessel at 400 °C contains the following equilibrium concentrations: N2, 1.00 M; H2, 0.50 M; and NH3,
0.25 M. How many moles of hydrogen must be removed from the vessel to increase the concentration of nitrogen to 1.1 M? The equilibrium reaction is
N2(g) + 3H2(g) ⇌ 2NH3(g)
-

