Private: Chapter Eleven
Electrolytes (11.2)
By the end of this module, you will be able to:
- Define and give examples of electrolytes
- Distinguish between the physical and chemical changes that accompany dissolution of ionic and covalent electrolytes
- Relate electrolyte strength to solute-solvent attractive forces
When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. These substances constitute an important class of compounds called electrolytes. Substances that do not yield ions when dissolved are called nonelectrolytes. If the physical or chemical process that generates the ions is essentially 100% efficient (all of the dissolved compound yields ions), then the substance is known as a strong electrolyte. If only a relatively small fraction of the dissolved substance undergoes the ion-producing process, it is called a weak electrolyte.
Substances may be identified as strong, weak, or nonelectrolytes by measuring the electrical conductance of an aqueous solution containing the substance. To conduct electricity, a substance must contain freely mobile, charged species. Most familiar is the conduction of electricity through metallic wires, in which case the mobile, charged entities are electrons. Solutions may also conduct electricity if they contain dissolved ions, with conductivity increasing as ion concentration increases. Applying a voltage to electrodes immersed in a solution permits assessment of the relative concentration of dissolved ions, either quantitatively, by measuring the electrical current flow, or qualitatively, by observing the brightness of a light bulb included in the circuit (Figure 11.6).
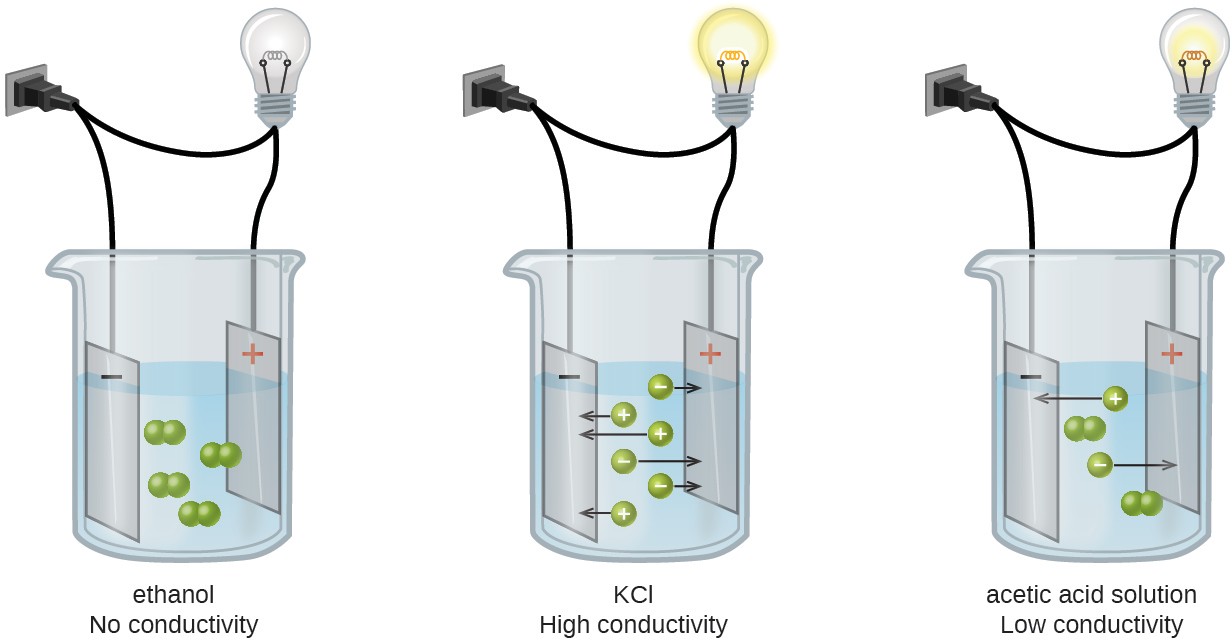
Ionic Electrolytes
Water and other polar molecules are attracted to ions, as shown in Figure 11.7. The electrostatic attraction between an ion and a molecule with a dipole is called an ion-dipole attraction. These attractions play an important role in the dissolution of ionic compounds in water.
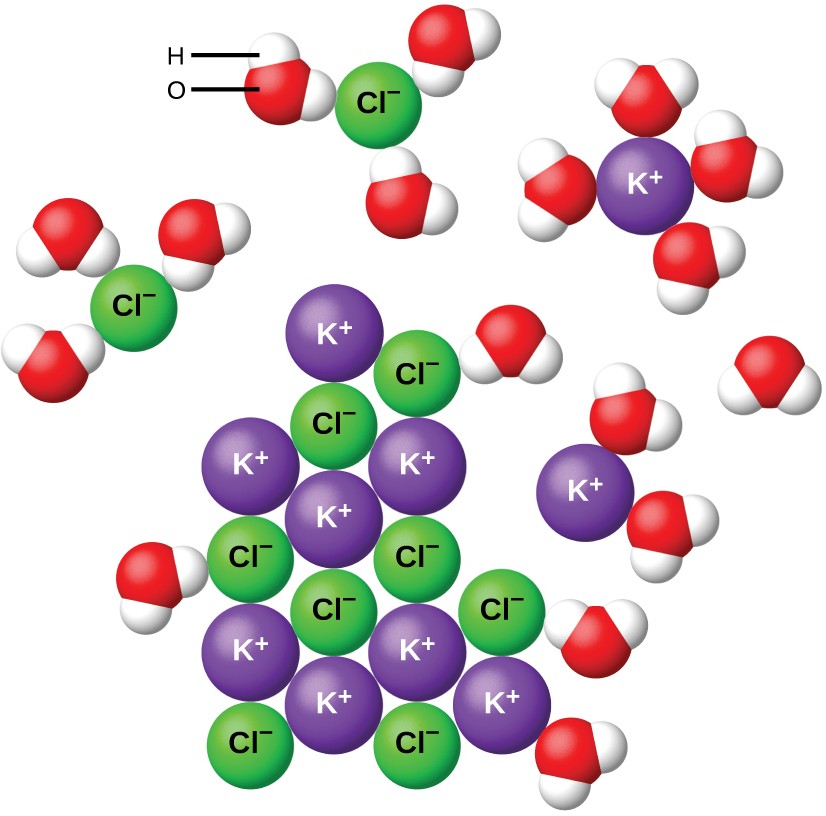
When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and solvate the ions, reducing the strong electrostatic forces between them. This process represents a physical change known as dissociation. Under most conditions, ionic compounds will dissociate nearly completely when dissolved, and so they are classified as strong electrolytes. Even sparingly, soluble ionic compounds are strong electrolytes, since the small amount that does dissolve will dissociate completely.
Consider what happens at the microscopic level when solid KCl is added to water. Ion-dipole forces attract the positive (hydrogen) end of the polar water molecules to the negative chloride ions at the surface of the solid, and they attract the negative (oxygen) ends to the positive potassium ions. The water molecules surround individual K+ and Cl− ions, reducing the strong interionic forces that bind the ions together and letting them move off into solution as solvated ions, as Figure 11.7 shows. Overcoming the electrostatic attraction permits the independent motion of each hydrated ion in a dilute solution as the ions transition from fixed positions in the undissolved compound to widely dispersed, solvated ions in solution.
Covalent Electrolytes
Pure water is an extremely poor conductor of electricity because it is only very slightly ionized—only about two out of every 1 billion molecules ionize at 25 °C. Water ionizes when one molecule of water gives up a proton (H+ ion) to another molecule of water, yielding hydronium and hydroxide ions.
H2O(l) + H2O(l) ⇌ H3O+(aq) + OH−(aq)
In some cases, solutions prepared from covalent compounds conduct electricity because the solute molecules react chemically with the solvent to produce ions. For example, pure hydrogen chloride is a gas consisting of covalent HCl
molecules. This gas contains no ions. However, an aqueous solution of HCl is a very good conductor, indicating that an appreciable concentration of ions exists within the solution.
3
Because HCl is an acid, its molecules react with water, transferring H+ ions to form hydronium ions (H O+) and chloride ions (Cl−):
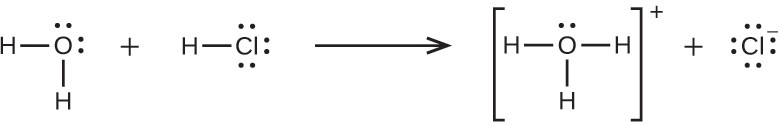
This reaction is essentially 100% complete for HCl (i.e., it is a strong acid and, consequently, a strong electrolyte). Likewise, weak acids and bases that only react partially generate relatively low concentrations of ions when dissolved in water and are classified as weak electrolytes. The reader may wish to review the discussion of strong and weak acids provided in the earlier chapter of this text on reaction classes and stoichiometry.

