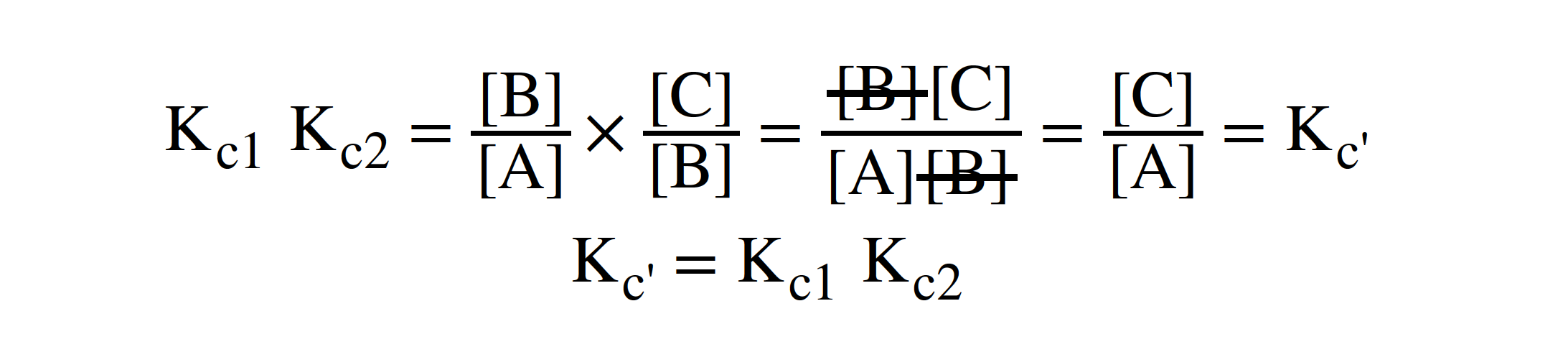Private: Chapter Thirteen
Equilibrium Constants (13.2)
OpenStax
Learning Objectives
By the end of this section, you will be able to:
- Derive reaction quotients from chemical equations representing homogeneous and heterogeneous reactions
- Calculate values of reaction quotients and equilibrium constants, using concentrations and pressures
- Relate the magnitude of an equilibrium constant to properties of the chemical system
The status of a reversible reaction is conveniently assessed by evaluating its reaction quotient (Q). For a reversible reaction described by
mA + nB + ⇌ xC + yD
the reaction quotient is derived directly from the stoichiometry of the balanced equation as
[latex]Q_c = \frac{[\text{C}]^x[\text{D}]^y}{[\text{A}]^m[\text{B}]^n}[/latex]
where the subscript c denotes the use of molar concentrations in the expression. If the reactants and products are gaseous, a reaction quotient may be similarly derived using partial pressures:
[latex]Q_p = \frac{\text{P}^x_C \text{P}^y_D}{\text{P}^m_A \text{P}^n_B}[/latex]
Note that the reaction quotient equations above are a simplification of more rigorous expressions that use relative values for concentrations and pressures rather than absolute values. These relative concentration and pressure values are dimensionless (they have no units); consequently, so are the reaction quotients. For purposes of this introductory text, it will suffice to use the simplified equations and to disregard units when computing Q. In most cases, this will introduce only modest errors in calculations involving reaction quotients.
Example 13.1
Writing Reaction Quotient Expressions
Write the concentration-based reaction quotient expression for each of the following reactions:
(a) 3O2(g) ⇌ 2O3(g)
(b) N2(g) + 3H2(g) ⇌ 2NH3(g)
(c) 4NH3(g) + 7O2(g) ⇌ 4NO2(g) + 6H2O(g)
Solution
(a) [latex]Q_c = \frac{[\text{O}_3]^2}{[\text{O}_2]^3}[/latex]
(b) [latex]Q_c = \frac{[\text{NH}_3]^2}{[\text{N}_2][\text{H}_2]^3}[/latex]
(c) [latex]Q_c = \frac{[\text{NO}_2]^4[\text{H}_2\text{O}]^6}{[\text{NH}_3]^4[\text{O}_2]^7}[/latex]
Check Your Learning
Write the concentration-based reaction quotient expression for each of the following reactions:
(a) 2SO2(g) + O2(g) ⇌ 2SO3(g)
(b) C4H8(g) ⇌ 2C2 H4(g)
(c) 2C4 H10(g) + 13O2(g) ⇌ 8CO2(g) + 10H2O(g)
Answer: (a) [latex]Q_c = \frac{[\text{SO}_3]^2}{[\text{SO}_2]^2[O_2]}[/latex] ; (b) [latex]Q_c = \frac{[\text{C}_2\text{H}_4]^2}{[\text{C}_4 \text{H}_8]}[/latex] ; (c) [latex]Q_c = \frac{[\text{CO}_2]^8[\text{H}_2\text{O}]^10}{[\text{C}_4\text{H}_10]^2[\text{O}_2]^13}[/latex]
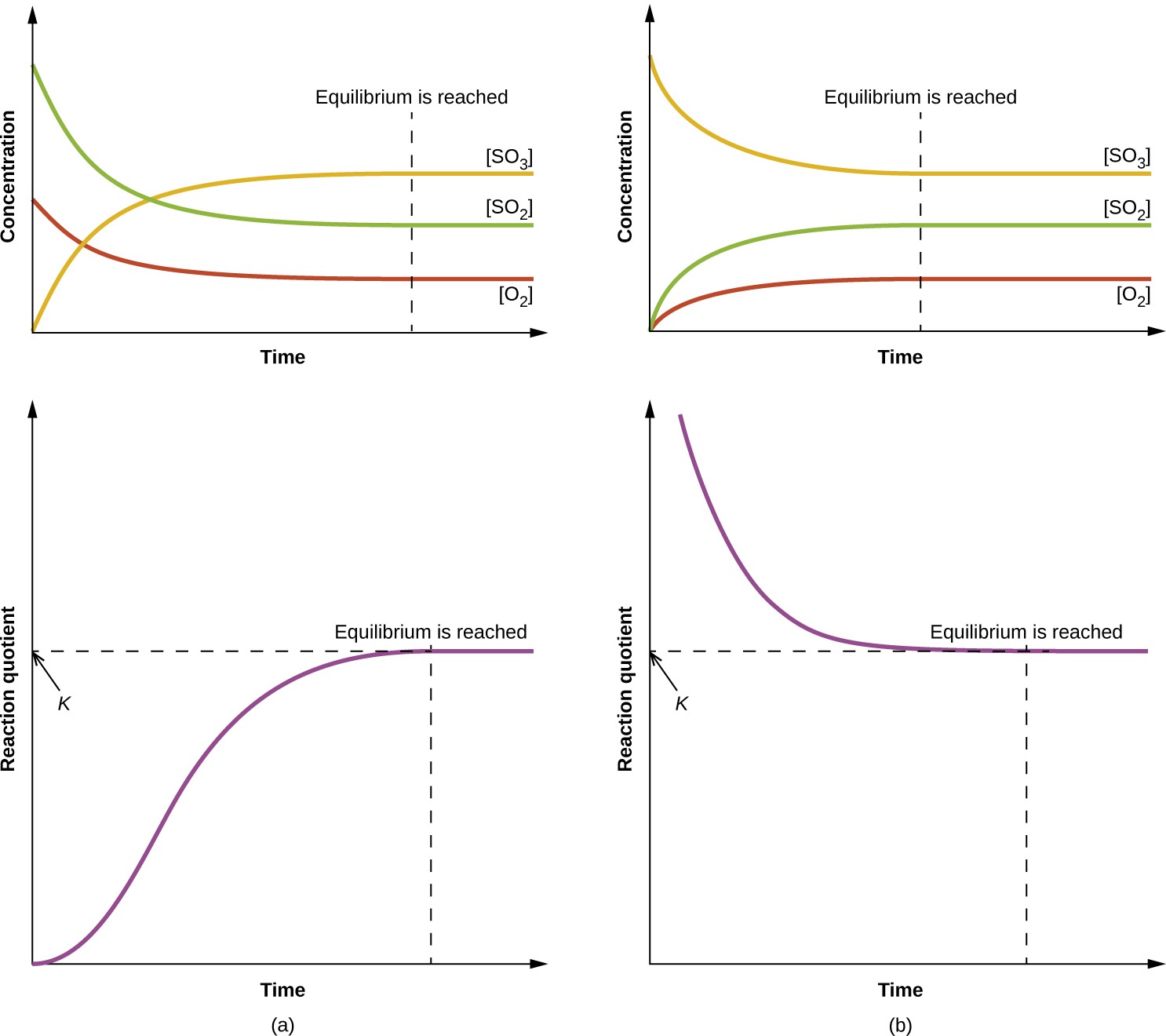
Figure 13.5 Changes in concentrations and Qc for a chemical equilibrium achieved beginning with (a) a mixture of reactants only and (b) products only.
The numerical value of Q varies as a reaction proceeds towards equilibrium; therefore, it can serve as a useful indicator of the reaction’s status. To illustrate this point, consider the oxidation of sulfur dioxide:
2SO2(g) + O2(g) ⇌ 2SO3(g)
Two different experimental scenarios are depicted in Figure 13.5, one in which this reaction is initiated with a mixture of reactants only, SO2 and O2, and another that begins with only product, SO3. For the reaction that begins with a mixture of reactants only, Q is initially equal to zero:
[latex]Q_c = \frac{[\text{SO}_3]^2}{[\text{SO}_2]^2[O_2]} = \frac{0_2}{[\text{SO}_2]^2[O_2]}[/latex] = 0
As the reaction proceeds toward equilibrium in the forward direction, reactant concentrations decrease (as does the denominator of Qc), product concentration increases (as does the numerator of Qc), and the reaction quotient consequently increases. When equilibrium is achieved, the concentrations of reactants and product remain constant, as does the value of Qc.
If the reaction begins with only product present, the value of Qc is initially undefined (immeasurably large, or infinite):
[latex]Q_c = \frac{[\text{SO}_3]^2}{[\text{SO}_2]^2[O_2]} = \frac{[\text{SO}_3]^2}{\text{0 → ∞}}[/latex]
In this case, the reaction proceeds toward equilibrium in the reverse direction. The product concentration and the numerator of Qc decrease with time, the reactant concentrations and the denominator of Qc increase, and the reaction quotient consequently decreases until it becomes constant at equilibrium.
The constant value of Q exhibited by a system at equilibrium is called the equilibrium constant, K:
K ≡ Q at equilibrium
Comparison of the data plots in Figure 13.5 shows that both experimental scenarios resulted in the same value for the equilibrium constant. This is a general observation for all equilibrium systems, known as the law of mass action: At a given temperature, the reaction quotient for a system at equilibrium is constant.
Example 13.2
Evaluating a Reaction Quotient
Gaseous nitrogen dioxide forms dinitrogen tetroxide according to this equation:
2NO2(g) ⇌ N2 O4(g)
When 0.10 mol NO2 is added to a 1.0-L flask at 25 °C, the concentration changes so that at equilibrium, [NO2] = 0.016 M and [N2O4] = 0.042 M.
- What is the value of the reaction quotient before any reaction occurs?
- What is the value of the equilibrium constant for the reaction?
Solution
As for all equilibrium calculations in this text, use the simplified equations for Q and K and disregard any concentration or pressure units, as noted previously in this section.
- Before any product is formed, [NO2] = [latex]\frac{0.10 \text{mol}}{1.0\text{L}}[/latex] = 0.10 M, and [N2O4] = 0 M. Thus, [latex]Q_c = \frac{[\text{N}_2\text{O}_4]}{[\text{NO}_2]^2} = \frac{0}{0.10^2} = 0[/latex]
- At equilibrium, Kc = Qc = [latex]\frac{[\text{N}_2\text{O}_4]}{[\text{NO}_2]^2} = \frac{0.042}{0.016^2}[/latex] = 1.6 x 102
Check Your Learning
For the reaction 2SO2(g) + O2(g) ⇌ 2SO3(g), the concentrations at equilibrium are [SO2] = 0.90 M, [O2] = 0.35 M, and [SO3] = 1.1 M. What is the value of the equilibrium constant, Kc?
Answer: Kc = 4.3
By its definition, the magnitude of an equilibrium constant explicitly reflects the composition of a reaction mixture at equilibrium, and it may be interpreted with regard to the extent of the forward reaction. A reaction exhibiting a large K will reach equilibrium when most of the reactant has been converted to product, whereas a small K indicates the reaction achieves equilibrium after very little reactant has been converted. It’s important to keep in mind that the magnitude of K does not indicate how rapidly or slowly equilibrium will be reached. Some equilibria are established so quickly as to be nearly instantaneous, and others so slowly that no perceptible change is observed over the course of days, years, or longer.
The equilibrium constant for a reaction can be used to predict the behavior of mixtures containing its reactants and/or products. As demonstrated by the sulfur dioxide oxidation process described above, a chemical reaction will proceed in whatever direction is necessary to achieve equilibrium. Comparing Q to K for an equilibrium system of interest allows prediction of what reaction (forward or reverse), if any, will occur.
To further illustrate this important point, consider the reversible reaction shown below:
CO(g) + H2 O(g) ⇌ CO2(g) + H2(g) Kc = 0.640 T = 800 °C
The bar charts in Figure 13.6 represent changes in reactant and product concentrations for three different reaction mixtures. The reaction quotients for mixtures 1 and 3 are initially lesser than the reaction’s equilibrium constant, so each of these mixtures will experience a net forward reaction to achieve equilibrium. The reaction quotient for mixture 2 is initially greater than the equilibrium constant, so this mixture will proceed in the reverse direction until equilibrium is established.
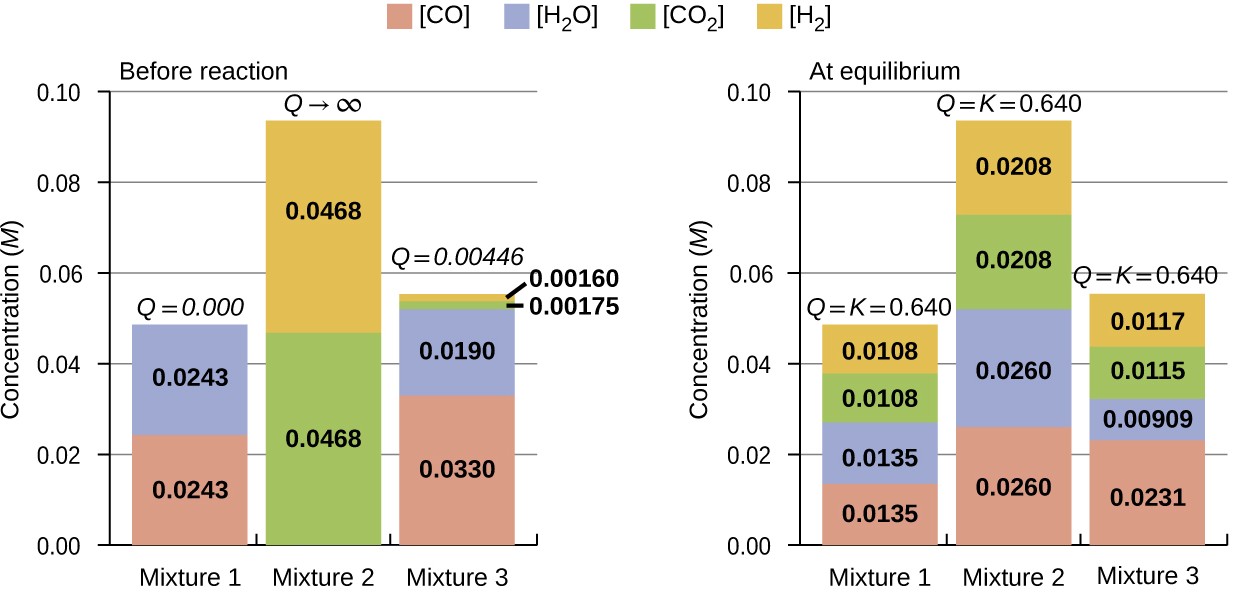
Figure 13.6 Compositions of three mixtures before (Qc ≠ Kc) and after (Qc = Kc) equilibrium is established for the reaction CO(g) + H2O(g) ⇌ CO2(g) + H2(g).
Example 13.3
Predicting the Direction of Reaction
Given here are the starting concentrations of reactants and products for three experiments involving this reaction:
CO(g) + H2O(g) ⇌ CO2(g) + H2(g)
Kc = 0.64
Determine in which direction the reaction proceeds as it goes to equilibrium in each of the three experiments shown.
|
Reactants/Products |
Experiment 1 |
Experiment 2 |
Experiment 3 |
|
[CO]i |
0.020 M |
0.011 M |
0.0094 M |
|
[H2O]i |
0.020 M |
0.0011 M |
0.0025 M |
|
[CO2]i |
0.0040 M |
0.037 M |
0.0015 M |
|
[H2]i |
0.0040 M |
0.046 M |
0.0076 M |
Solution
Experiment 1:
[latex]Q_c = \frac{[\text{CO}_2][\text{H}_2]}{[\text{CO}][\text{H}_2\text{O}]} = \frac{(0.0040)(0.0040)}{(0.020)(0.020)} = 0.040[/latex]
Qc < Kc (0.040 < 0.64)
The reaction will proceed in the forward direction.
Experiment 2:
[latex]Q_c = \frac{[\text{CO}_2][\text{H}_2]}{[\text{CO}][\text{H}_2\text{O}]} = \frac{(0.037)(0.046)}{(0.011)(0.011)}[/latex] = 1.4 x 102
Qc < Kc (140 > 0.64)
The reaction will proceed in the reverse direction.
Experiment 3:
[latex]Q_c = \frac{[\text{CO}_2][\text{H}_2]}{[\text{CO}][\text{H}_2\text{O}]} = \frac{(0.0015)(0.0076)}{(0.0094)(0.0025)}[/latex] = 1.4 x 102
Qc < Kc (0.48 < 0.64)
The reaction will proceed in the forward direction.
Check Your Learning
Calculate the reaction quotient and determine the direction in which each of the following reactions will proceed to reach equilibrium.
A 1.00-L flask containing 0.0500 mol of NO(g), 0.0155 mol of Cl2(g), and 0.500 mol of NOCl:
2NO(g) + Cl2(g) ⇌ 2NOCl(g) Kc = 4.6 × 104
A 5.0-L flask containing 17 g of NH3, 14 g of N2, and 12 g of H2:
N2(g) + 3H2(g) ⇌ 2NH3(g) Kc = 0.060
A 2.00-L flask containing 230 g of SO3(g):
2SO3(g) ⇌ 2SO2(g) Kc = 0.230
Answer: (a) Qc = 6.45 × 103, forward. (b) Qc = 0.23, reverse. (c) Qc = 0, forward
Homogeneous Equilibria
A homogeneous equilibrium is one in which all reactants and products (and any catalysts, if applicable) are present in the same phase. By this definition, homogeneous equilibria take place in solutions. These solutions are most commonly either liquid or gaseous phases, as shown by the examples below:
C2H2(aq) + 2Br2(aq) ⇌ C2H2Br4(aq) Kc = [latex]\frac{[\text{C}_2\text{H}_2\text{Br}_4]}{[\text{C}_2\text{H}_2][\text{Br}_2]^2}[/latex]
I2(aq) + I -(aq) ⇌ I3-(aq) Kc = [latex]\frac{[\text{I}_3 ^-]}{[\text{I}_2][\text{I}^-]}[/latex]
HF(aq) + H2O(l) ⇌ H3O+(aq) + F-(aq) Kc = [latex]\frac{[\text{H}_3\text{O}^+][\text{F}^-]}{[\text{HF}]}[/latex]
NH3(aq) + H2O(l) ⇌ NH4+(aq) + OH-(aq) Kc = [latex]\frac{[\text{NH}_4^+][\text{OH}^-]}{[\text{NH}_3]}[/latex]
These examples all involve aqueous solutions, those in which water functions as the solvent. In the last two examples, water also functions as a reactant, but its concentration is not included in the reaction quotient. The reason for this omission is related to the more rigorous form of the Q (or K) expression mentioned previously in this chapter, in which relative concentrations for liquids and solids are equal to 1 and needn’t be included. Consequently, reaction quotients include concentration or pressure terms only for gaseous and solute species.
The equilibria below all involve gas-phase solutions:
C2H6(g) ⇌ C2H4(g) + H2(g) Kc = [latex]\frac{[\text{C}_2\text{H}_4][\text{H}_2]}{[\text{C}_2\text{H}_6]}[/latex]
3O2(g) ⇌ 2O3(g) Kc = [latex]\frac{[\text{O}_3]^2}{[\text{O}_2]^3}[/latex]
N2(g) + 3H2(g) ⇌ 2NH3(g) Kc = [latex]\frac{[\text{NH}_3]^2}{[\text{N}_2][\text{H}_2]^3}[/latex]
C3H8(g) + 5O2(g) ⇌ 2CO2(g) + 4H2O(g) Kc = [latex]\frac{[\text{CO}_2]^3[\text{H}_2\text{O}]^4}{[\text{C}_3\text{H}_8][\text{O}_2]^5}[/latex]
For gas-phase solutions, the equilibrium constant may be expressed in terms of either the molar concentrations (Kc) or partial pressures (Kp) of the reactants and products. A relation between these two K values may be simply derived from the ideal gas equation and the definition of molarity:
where P is partial pressure, V is volume, n is molar amount, R is the gas constant, T is temperature, and M is molar concentration.
For the gas-phase reaction mA + nB ⇌ xC + yD:
[latex]\begin{array}{r @{{}={}} l} K_P = \frac{(P_C)^x(P_D)^y}{(P_A)^m(P_B)^n} \\[0.5em] = \frac{([\text{C}]\;\times\;RT)^x([\text{D}]\;\times\;RT)^y}{([\text{A}]\;\times\;RT)^m([\text{B}]\;\times\;RT)^n} \\[0.5em] = \frac{[\text{C}]^x[\text{D}]^y}{[\text{A}]^m[\text{B}]^n}\;\times\;\frac{(RT)^{x+y}}{(RT)^{m+n}} \\[0.5em] = K_c(RT)^{(x+y)\;-\;(m+n)} \\[0.5em] = K_c(RT)^{{\Delta}n} \end{array}[/latex]
And so, the relationship between Kc and KP is
Kp = Kc(RT) [latex]^{{\Delta}n}[/latex]
where Δn is the difference in the molar amounts of product and reactant gases, in this case:
Δn = (x+y) − (m+n)
Example 13.4
Calculation of KP
Write the equations relating Kc to KP for each of the following reactions:
(a) C2H6(g) ⇌ C2H4(g) + H2(g)
(b) CO(g) + H2O(g) ⇌ CO2(g) + H2(g)
(c) N2(g) + 3H2(g) ⇌ 2NH3(g)
(d) Kc is equal to 0.28 for the following reaction at 900 °C:
CS2(g) + 4H2(g) ⇌ CH4(g) + 2H2S(g)
What is KP at this temperature?
Solution
(a) Δn = (2) − (1) = 1
KP = Kc (RT)Δn = Kc (RT)1 = Kc (RT)
(b) Δn = (2) − (2) = 0
KP = Kc (RT)Δn = Kc (RT)0 = Kc
(c) Δn = (2) − (1 + 3) = −2
KP = Kc (RT)Δn = Kc(RT)-2 = [latex]\frac{\text{K}_c}{(\text{RT})^2}[/latex]
(d) KP = Kc (RT)Δn = (0.28)[(0.0821)(1173)]−2 = 3.0 × 10−5
Check Your Learning
Write the equations relating Kc to KP for each of the following reactions:
(a) 2SO2(g) + O2(g) ⇌ 2SO3(g)
(b) N2O4(g) ⇌ 2NO2(g)
(c) C3H8(g) + 5O2(g) ⇌ 3CO2(g) + 4H2O(g)
(d) At 227 °C, the following reaction has Kc = 0.0952:
CH3OH(g) ⇌ CO(g) + 2H2(g)
What would be the value of KP at this temperature?
Answer: (a) KP = Kc (RT)−1; (b) KP = Kc (RT); (c) KP = Kc (RT); (d) 160 or 1.6 × 102
Heterogeneous Equilibria
A heterogeneous equilibrium involves reactants and products in two or more different phases, as illustrated by the following examples:
PbCl2(s) ⇌ Pb2+(aq) + 2Cl−(aq) Kc = [Pb2+][Cl−]2
CaO(s) + CO2(g) ⇌ CaCO3(s) Kc = [latex]\frac{1}{[\text{CO}_2]}[/latex]
C(s) + 2S(g) ⇌ CS2(g) Kc = [latex]\frac{[\text{CS}_2]}{[\text{S}]^2}[/latex]
Br2(l) ⇌ Br2(g) Kc = [Br2(g)]
Again, note that concentration terms are only included for gaseous and solute species, as discussed previously.
Two of the above examples include terms for gaseous species only in their equilibrium constants, and so Kp expressions may also be written:
[latex]\begin{array}{r @{{}\rightleftharpoons{}} ll} \text{CaO}(s)\;+\;\text{CO}_2(g) & \text{CaCO}_3(s) & K_P = \frac{1}{P_{\text{CO}_2}} \\[0.5em] \text{C}(s)\;+\;2\text{S}(g) & \text{CS}_2(g) & K_P = \frac{P_{\text{CS}_2}}{(P_{\text{S}})^2} \end{array}[/latex]
Coupled Equilibria
The equilibrium systems discussed so far have all been relatively simple, involving just single reversible reactions. Many systems, however, involve two or more coupled equilibrium reactions, those which have in common one or more reactant or product species. Since the law of mass action allows for a straightforward derivation of equilibrium constant expressions from balanced chemical equations, the K value for a system involving coupled equilibria can be related to the K values of the individual reactions. Three basic manipulations are involved in this approach, as described below.
- Changing the direction of a chemical equation essentially swaps the identities of “reactants” and “products,” and so the equilibrium constant for the reversed equation is simply the reciprocal of that for the forward equation.
A ⇌ B Kc = [latex]\frac{[B]}{[A]}[/latex]
B ⇌ A Kc' = [latex]\frac{[A]}{[B]}[/latex]
Kc' = [latex]\frac{1}{\text{K}_c}[/latex]
- Changing the stoichiometric coefficients in an equation by some factor x results in an exponential change in the equilibrium constant by that same factor:
A ⇌ B Kc = [latex]\frac{[B]}{[A]}[/latex]
xA ⇌ xB Kc' = [latex]\frac{[B]^x}{[A]^x}[/latex]
Kc' = Kc'x
- Adding two or more equilibrium equations together yields an overall equation whose equilibrium constant is the mathematical product of the individual reaction’s K values:
A ⇌ B Kc1 = [latex]\frac{[B]}{[A]}[/latex]
B ⇌ C Kc2 = [latex]\frac{[C]}{[B]}[/latex]
The net reaction for these coupled equilibria is obtained by summing the two equilibrium equations and canceling any redundancies:
A + B ⇌ B + C
A + B ⇌ B + C
A ⇌ C Kc' = [latex]\frac{[C]}{[A]}[/latex]
Comparing the equilibrium constant for the net reaction to those for the two coupled equilibrium reactions reveals the following relationship:
Example 13.5 demonstrates the use of this strategy in describing coupled equilibrium processes.
Example 13.5
Equilibrium Constants for Coupled Reactions
A mixture containing nitrogen, hydrogen, and iodine established the following equilibrium at 400 °C:
2NH3(g) + 3I2(g) ⇌ N(g)2 + 6HI(g)
Use the information below to calculate Kc for this reaction.
N2(g) + 3H2(g) ⇌ 2NH3(g) Kc1 = 0.50 at 400 ° C
H2(g) + I2(g) ⇌ 2HI(g) Kc2 = 50 at 400 ° C
Solution
The equilibrium equation of interest and its K value may be derived from the equations for the two coupled reactions as follows.
Reverse the first coupled reaction equation:
2NH3(g) ⇌ N2(g) + 3H2(g) Kc1' = [latex]\frac{1}{\text{K}_{c1}} = \frac{1}{0.50}[/latex] = 2.0
Multiply the second coupled reaction by 3:
3H2(g) + 3I2(g) ⇌ 6HI(g) Kc2' = K3c2 = 503 = 1.2 × 105
Finally, add the two revised equations:
2NH3(g) + 3H2(g) + 3I2(g) ⇌ N2(g) + 3H2(g) + 6HI(g)
2NH3(g) + 3I2(g) ⇌ N2(g) + 6HI(g)
Kc = Kc1' Kc2' = (2.0)(1.2 × 105) = 2.5 × 105
Check Your Learning
Use the provided information to calculate Kc for the following reaction at 550 °C:
H2(g) + CO2(g) ⇌ CO(g) + H2O(g) Kc = ?
CoO(s) + CO(g) ⇌ Co(s) + CO2(g) Kc1 = 490
CoO(s) + H2(g) ⇌ Co(s) + H2O(g) Kc1 = 67
Answer: Kc = 0.14
Key Concepts and Summary
For any reaction that is at equilibrium, the reaction quotient Q is equal to the equilibrium constant K for the reaction. If a reactant or product is a pure solid, a pure liquid, or the solvent in a dilute solution, the concentration of this component does not appear in the expression for the equilibrium constant. At equilibrium, the values of the concentrations of the reactants and products are constant. Their particular values may vary depending on conditions, but the value of the reaction quotient will always equal K (Kc when using concentrations or KP when using partial pressures).
A homogeneous equilibrium is an equilibrium in which all components are in the same phase. A heterogeneous equilibrium is an equilibrium in which components are in two or more phases. We can decide whether a reaction is at equilibrium by comparing the reaction quotient with the equilibrium constant for the reaction.
Key Equations
- [latex]Q = \frac{[\text{C}]^x[\text{D}]^y}{[\text{A}]^m[\text{B}]^n}\;\;\;\;\;\;\;\text{where}\;m\text{A}\;+\;n\text{B}\;{\rightleftharpoons}\;x\text{C}\;+\;y\text{D}[/latex]
- [latex]Q_P = \frac{(P_C)^x(P_D)^y}{(P_A)^m(P_B)^n}\;\;\;\;\;\;\;\text{where}\;m\text{A}\;+\;n\text{B}\;{\rightleftharpoons}\;x\text{C}\;+\;y\text{D}[/latex]
- P = MRT
- KP = Kc (RT)Δn
Chemistry End of Chapter Exercises
- Explain why there may be an infinite number of values for the reaction quotient of a reaction at a given temperature but there can be only one value for the equilibrium constant at that temperature.
- Explain why an equilibrium between Br2(l) and Br2(g) would not be established if the container were not a closed vessel shown in Figure 4 in Chapter 13.1 Chemical Equilibria.
- If you observe the following reaction at equilibrium, is it possible to tell whether the reaction started with pure NO2 or with pure N2O4?
[latex]2\text{NO}_2(g)\;{\rightleftharpoons}\;\text{N}_2\text{O}_4(g)[/latex] - Among the solubility rules previously discussed is the statement: All chlorides are soluble except Hg2Cl2, AgCl, PbCl2, and CuCl.
(a) Write the expression for the equilibrium constant for the reaction represented by the equation [latex]\text{AgCl}(s)\;{\rightleftharpoons}\;\text{Ag}^{+}(aq)\;+\;\text{Cl}^{-}(aq)[/latex]. Is Kc > 1, < 1, or ≈ 1? Explain your answer.
(b) Write the expression for the equilibrium constant for the reaction represented by the equation [latex]\text{Pb}^{2+}(aq)\;+\;2\text{Cl}^{-}(aq)\;{\rightleftharpoons}\;\text{PbCl}_2(s)[/latex]. Is Kc > 1, < 1, or ≈ 1? Explain your answer.
- Among the solubility rules previously discussed is the statement: Carbonates, phosphates, borates, and arsenates—except those of the ammonium ion and the alkali metals—are insoluble.
(a) Write the expression for the equilibrium constant for the reaction represented by the equation [latex]\text{CaCO}_3(s)\;{\rightleftharpoons}\;\text{Ca}^{2+}(aq)\;+\;\text{CO}_3^{\;\;-}(aq)[/latex]. Is Kc > 1, < 1, or ≈ 1? Explain your answer.
(b) Write the expression for the equilibrium constant for the reaction represented by the equation [latex]3\text{Ba}^{2+}(aq)\;+\;2\text{PO}_4^{\;\;3-}(aq)\;{\rightleftharpoons}\;\text{Ba}_3(\text{PO}_4)_2(s)[/latex]. Is Kc > 1, < 1, or ≈ 1? Explain your answer.
- Benzene is one of the compounds used as octane enhancers in unleaded gasoline. It is manufactured by the catalytic conversion of acetylene to benzene: [latex]3\text{C}_2\text{H}_2(g)\;{\longrightarrow}\;\text{C}_6\text{H}_6(g)[/latex]. Which value of Kc would make this reaction most useful commercially? Kc ≈ 0.01, Kc ≈ 1, or Kc ≈ 10. Explain your answer.
- Show that the complete chemical equation, the total ionic equation, and the net ionic equation for the reaction represented by the equation [latex]\text{KI}(aq)\;+\;\text{I}_2(aq)\;{\rightleftharpoons}\;\text{KI}_3(aq)[/latex] give the same expression for the reaction quotient. KI3 is composed of the ions K+ and [latex]\text{I}_3^{\;\;-}[/latex].
- For a titration to be effective, the reaction must be rapid and the yield of the reaction must essentially be 100%. Is Kc > 1, < 1, or ≈ 1 for a titration reaction?
- For a precipitation reaction to be useful in a gravimetric analysis, the product of the reaction must be insoluble. Is Kc > 1, < 1, or ≈ 1 for a useful precipitation reaction?
- Write the mathematical expression for the reaction quotient, Qc, for each of the following reactions:
(a) [latex]\text{CH}_4(g)\;+\;\text{Cl}_2(g)\;{\rightleftharpoons}\;\text{CH}_3\text{Cl}(g)\;+\;\text{HCl}(g)[/latex]
(b) [latex]\text{N}_2(g)\;+\;\text{O}_2(g)\;{\rightleftharpoons}\;2\text{NO}(g)[/latex]
(c) [latex]2\text{SO}_2(g)\;+\;\text{O}_2(g)\;{\rightleftharpoons}\;2\text{SO}_3(g)[/latex]
(d) [latex]\text{BaSO}_3(s)\;{\rightleftharpoons}\;\text{BaO}(s)\;+\;\text{SO}_2(g)[/latex]
(e) [latex]\text{P}_4(g)\;+\;5\text{O}_2(g)\;{\rightleftharpoons}\;\text{P}_4\text{O}_{10}(s)[/latex]
(f) [latex]\text{Br}_2(g)\;{\rightleftharpoons}\;2\text{Br}(g)[/latex]
(g) [latex]\text{CH}_4(g)\;+\;2\text{O}_2(g)\;{\rightleftharpoons}\;\text{CO}_2(g)\;+\;2\text{H}_2\text{O}(l)[/latex]
(h) [latex]\text{CuSO}_4{\cdot}5\text{H}_2\text{O}(s)\;{\rightleftharpoons}\;\text{CuSO}_4(s)\;+\;5\text{H}_2\text{O}(g)[/latex]
- Write the mathematical expression for the reaction quotient, Qc, for each of the following reactions:
(a) [latex]\text{N}_2(g)\;+\;3\text{H}_2(g)\;{\rightleftharpoons}\;2\text{NH}_3(g)[/latex]
(b) [latex]4\text{NH}_3(g)\;+\;5\text{O}_2(g)\;{\rightleftharpoons}\;4\text{NO}(g)\;+\;6\text{H}_2\text{O}(g)[/latex]
(c) [latex]\text{N}_2\text{O}_4(g)\;{\rightleftharpoons}\;2\text{NO}_2(g)[/latex]
(d) [latex]\text{CO}_2(g)\;+\;\text{H}_2(g)\;{\rightleftharpoons}\;\text{CO}(g)\;+\;\text{H}_2\text{O}(g)[/latex]
(e) [latex]\text{NH}_4\text{Cl}(s)\;{\rightleftharpoons}\;\text{NH}_3(g)\;+\;\text{HCl}(g)[/latex]
(f) [latex]2\text{Pb(NO}_3)_2(s)\;{\rightleftharpoons}\;2\text{PbO}(s)\;+\;4\text{NO}_2(g)\;+\;\text{O}_2(g)[/latex]
(g) [latex]2\text{H}_2(g)\;+\;\text{O}_2(g)\;{\rightleftharpoons}\;2\text{H}_2\text{O}(l)[/latex]
(h) [latex]\text{S}_8(g)\;{\rightleftharpoons}\;8\text{S}(g)[/latex]
- The initial concentrations or pressures of reactants and products are given for each of the following systems. Calculate the reaction quotient and determine the direction in which each system will proceed to reach equilibrium.
(a) [latex]2\text{NH}_3(g)\;{\rightleftharpoons}\;\text{N}_2(g)\;+\;3\text{H}_2(g)\;\;\;\;\;\;\;K_c = 17[/latex]; [NH3] = 0.20 M, [N2] = 1.00 M, [H2] = 1.00 M
(b) [latex]2\text{NH}_3(g)\;{\rightleftharpoons}\;\text{N}_2(g)\;+\;3\text{H}_2(g)\;\;\;\;\;\;\;K_P = 6.8\;\times\;10^4[/latex]; initial pressures: NH3 = 3.0 atm, N2 = 2.0 atm, H2 = 1.0 atm
(c) [latex]2\text{SO}_3(g)\;{\rightleftharpoons}\;2\text{SO}_2(g)\;+\;\text{O}_2(g)\;\;\;\;\;\;\;K_c = 0.230[/latex]; [SO3] = 0.00 M, [SO2] = 1.00 M, [O2] = 1.00 M
(d) [latex]2\text{SO}_3(g)\;{\rightleftharpoons}\;2\text{SO}_2(g)\;+\;\text{O}_2(g)\;\;\;\;\;\;\;K_P = 16.5[/latex]; initial pressures: SO3 = 1.00 atm, SO2 = 1.00 atm, O2 = 1.00 atm
(e) [latex]2\text{NO}(g)\;+\;\text{Cl}_2(g)\;{\rightleftharpoons}\;2\text{NOCl}(g)\;\;\;\;\;\;\;K_c = 4.6\;\times\;10^4[/latex]; [NO] = 1.00 M, [Cl2] = 1.00 M, [NOCl] = 0 M
(f) [latex]\text{N}_2(g)\;+\;\text{O}_2(g)\;{\rightleftharpoons}\;2\text{NO}(g)\;\;\;\;\;\;\;K_P = 0.050[/latex]; initial pressures: NO = 10.0 atm, N2 = O2 = 5 atm
- The initial concentrations or pressures of reactants and products are given for each of the following systems. Calculate the reaction quotient and determine the direction in which each system will proceed to reach equilibrium.
(a) [latex]2\text{NH}_3(g)\;{\rightleftharpoons}\;\text{N}_2(g)\;+\;3\text{H}_2(g)\;\;\;\;\;\;\;K_c = 17[/latex]; [NH3] = 0.50 M, [N2] = 0.15 M, [H2] = 0.12 M
(b) [latex]2\text{NH}_3(g)\;{\rightleftharpoons}\;\text{N}_2(g)\;+\;3\text{H}_2(g)\;\;\;\;\;\;\;K_P = 6.8\;\times\;10^4[/latex]; initial pressures: NH3 = 2.00 atm, N2 = 10.00 atm, H2 = 10.00 atm
(c) [latex]2\text{SO}_3(g)\;{\rightleftharpoons}\;2\text{SO}_2(g)\;+\;\text{O}_2(g)\;\;\;\;\;\;\;K_c = 0.230[/latex]; [SO3] = 2.00 M, [SO2] = 2.00 M, [O2] = 2.00 M
(d) [latex]2\text{SO}_3(g)\;{\rightleftharpoons}\;2\text{SO}_2(g)\;+\;\text{O}_2(g)\;\;\;\;\;\;\;K_P = 6.5\;\text{atm}[/latex]; initial pressures: SO2 = 1.00 atm, O2 = 1.130 atm, SO3 = 0 atm
(e) [latex]2\text{NO}(g)\;+\;\text{Cl}_2(g)\;{\rightleftharpoons}\;2\text{NOCl}(g)\;\;\;\;\;\;\;K_P = 2.5\;\times\;10^3[/latex]; initial pressures: NO = 1.00 atm, Cl2 = 1.00 atm, NOCl = 0 atm
(f) [latex]\text{N}_2(g)\;+\;\text{O}_2(g)\;{\rightleftharpoons}\;2\text{NO}(g)\;\;\;\;\;\;\;K_c = 0.050[/latex]; [N2] = 0.100 M, [O2] = 0.200 M, [NO] = 1.00 M
- The following reaction has KP = 4.50 × 10−5 at 720 K.
[latex]\text{N}_2(g)\;+\;3\text{H}_2(g)\;{\rightleftharpoons}\;2\text{NH}_3(g)[/latex]If a reaction vessel is filled with each gas to the partial pressures listed, in which direction will it shift to reach equilibrium? P(NH3) = 93 atm, P(N2) = 48 atm, and P(H2) = 52
- Determine if the following system is at equilibrium. If not, in which direction will the system need to shift to reach equilibrium?
[latex]\text{SO}_2\text{Cl}_2(g)\;{\rightleftharpoons}\;\text{SO}_2(g)\;+\;\text{Cl}_2(g)[/latex][SO2Cl2] = 0.12 M, [Cl2] = 0.16 M and [SO2] = 0.050 M. Kc for the reaction is 0.078.
- Which of the systems described in Chapter 13.2 Chemistry End of Chapter Exercise 10 give homogeneous equilibria? Which give heterogeneous equilibria?
- Which of the systems described in Chapter 13.2 Chemistry End of Chapter Exercise 11 give homogeneous equilibria? Which give heterogeneous equilibria?
- For which of the reactions in Chapter 13.2 Chemistry End of Chapter Exercise 10 does Kc (calculated using concentrations) equal KP (calculated using pressures)?
- For which of the reactions in Chapter 13.2 Chemistry End of Chapter Exercise 11 does Kc (calculated using concentrations) equal KP (calculated using pressures)?
- Convert the values of Kc to values of KP or the values of KP to values of Kc.
(a) [latex]\text{N}_2(g)\;+\;3\text{H}_2(g)\;{\rightleftharpoons}\;2\text{NH}_3(g)\;\;\;\;\;\;\;K_c = 0.50\;\text{at}\;400\;^{\circ}\text{C}[/latex]
(b) [latex]\text{H}_2\;+\;\text{I}_2\;{\rightleftharpoons}\;2\text{HI}\;\;\;\;\;\;\;K_c = 50.2\;\text{at}\;448\;^{\circ}\text{C}[/latex]
(c)[latex]\text{Na}_2\text{SO}_4{\cdot}10\text{H}_2\text{O}(s)\;{\rightleftharpoons}\;\text{Na}_2\text{SO}_4(s)\;+\;10\text{H}_2\text{O}(g)\;\;\;\;\;\;\;K_P = 4.08\;\times\;10^{-25}\;\text{at}\;25\;^{\circ}\text{C}[/latex]
(d)[latex]\text{H}_2\text{O}(l)\;{\rightleftharpoons}\;\text{H}_2\text{O}(g)\;\;\;\;\;\;\;K_P = 0.122\;\text{at}\;50\;^{\circ}\text{C}[/latex]
- Convert the values of Kc to values of KP or the values of KP to values of Kc.
(a) [latex]\text{Cl}_2(g)\;+\;\text{Br}_2(g)\;{\rightleftharpoons}\;2\text{BrCl}(g)\;\;\;\;\;\;\;K_c = 4.7\;\times\;10^{-2}\;\text{at}\;25\;^{\circ}\text{C}[/latex]
(b) [latex]2\text{SO}_2(g)\;+\;\text{O}_2(g)\;{\rightleftharpoons}\;2\text{SO}_3(g)\;\;\;\;\;\;\;K_P = 48.2\;\text{at}\;500\;^{\circ}\text{C}[/latex]
(c) [latex]\text{CaCl}_2{\cdot}6\text{H}_2\text{O}(s)\;{\rightleftharpoons}\;\text{CaCl}_2(s)\;+\;6\text{H}_2\text{O}(g)\;\;\;\;\;\;\;K_P = 5.09\;\times\;10^{-44}\;\text{at}\;25\;^{\circ}\text{C}[/latex]
(d) [latex]\text{H}_2\text{O}(l)\;{\rightleftharpoons}\;\text{H}_2\text{O}(g)\;\;\;\;\;\;\;K_P = 0.196\;\text{at}\;60\;^{\circ}\text{C}[/latex]
- What is the value of the equilibrium constant expression for the change [latex]\text{H}_2\text{O}(l)\;{\rightleftharpoons}\;\text{H}_2\text{O}(g)[/latex] at 30 °C?
- Write the expression of the reaction quotient for the ionization of HOCN in water.
- Write the reaction quotient expression for the ionization of NH3 in water.
- What is the approximate value of the equilibrium constant KP for the change [latex]\text{C}_2\text{H}_5\text{OC}_2\text{H}_5(l)\;{\rightleftharpoons}\;\text{C}_2\text{H}_5\text{OC}_2\text{H}_5(g)[/latex] at 25 °C. (Vapor pressure was described in the previous chapter on liquids and solids; refer back to this chapter to find the relevant information needed to solve this problem.)