18.4 – B Lymphocytes and Humoral Immunity
Learning Objectives
- Describe the production and maturation of B cells
- Compare the structure of B-cell receptors and T-cell receptors
- Compare T-dependent and T-independent activation of B cells
- Compare the primary and secondary antibody responses
Humoral immunity refers to mechanisms of the adaptive immune defenses that are mediated by antibodies secreted by B lymphocytes, or B cells. This section will focus on B cells and discuss their production and maturation, receptors, and mechanisms of activation.
B Cell Production and Maturation
Like T cells, B cells are formed from multipotent hematopoietic stem cells (HSCs) in the bone marrow and follow a pathway through lymphoid stem cell and lymphoblast (see Figure 17.12). Unlike T cells, however, lymphoblasts destined to become B cells do not leave the bone marrow and travel to the thymus for maturation. Rather, eventual B cells continue to mature in the bone marrow.
The first step of B cell maturation is an assessment of the functionality of their antigen-binding receptors. This occurs through positive selection for B cells with normal functional receptors. A mechanism of negative selection is then used to eliminate self-reacting B cells and minimize the risk of autoimmunity. Negative selection of self-reacting B cells can involve elimination by apoptosis, editing or modification of the receptors so they are no longer self-reactive, or induction of anergy in the B cell. Immature B cells that pass the selection in the bone marrow then travel to the spleen for their final stages of maturation. There they become naïve mature B cells, i.e., mature B cells that have not yet been activated.
Check Your Understanding
- Compare the maturation of B cells with the maturation of T cells.
B-Cell Receptors
Like T cells, B cells possess antigen-specific receptors with diverse specificities. Although they rely on T cells for optimum function, B cells can be activated without help from T cells. B-cell receptors (BCRs) for naïve mature B cells are membrane-bound monomeric forms of IgD and IgM. They have two identical heavy chains and two identical light chains connected by disulfide bonds into a basic “Y” shape (Figure 18.20). The trunk of the Y-shaped molecule, the constant region of the two heavy chains, spans the B cell membrane. The two antigen-binding sites exposed to the exterior of the B cell are involved in the binding of specific pathogen epitopes to initiate the activation process. It is estimated that each naïve mature B cell has upwards of 100,000 BCRs on its membrane, and each of these BCRs has an identical epitope-binding specificity.
In order to be prepared to react to a wide range of microbial epitopes, B cells, like T cells, use genetic rearrangement of hundreds of gene segments to provide the necessary diversity of receptor specificities. The variable region of the BCR heavy chain is made up of V, D, and J segments, similar to the β chain of the TCR. The variable region of the BCR light chain is made up of V and J segments, similar to the α chain of the TCR. Genetic rearrangement of all possible combinations of V-J-D (heavy chain) and V-J (light chain) provides for millions of unique antigen-binding sites for the BCR and for the antibodies secreted after activation.
One important difference between BCRs and TCRs is the way they can interact with antigenic epitopes. Whereas TCRs can only interact with antigenic epitopes that are presented within the antigen-binding cleft of MHC I or MHC II, BCRs do not require antigen presentation with MHC; they can interact with epitopes on free antigens or with epitopes displayed on the surface of intact pathogens. Another important difference is that TCRs only recognize protein epitopes, whereas BCRs can recognize epitopes associated with different molecular classes (e.g., proteins, polysaccharides, lipopolysaccharides).
Activation of B cells occurs through different mechanisms depending on the molecular class of the antigen. Activation of a B cell by a protein antigen requires the B cell to function as an APC, presenting the protein epitopes with MHC II to helper T cells. Because of their dependence on T cells for activation of B cells, protein antigens are classified as T- dependent antigens. In contrast, polysaccharides, lipopolysaccharides, and other nonprotein antigens are considered T-independent antigens because they can activate B cells without antigen processing and presentation to T cells.
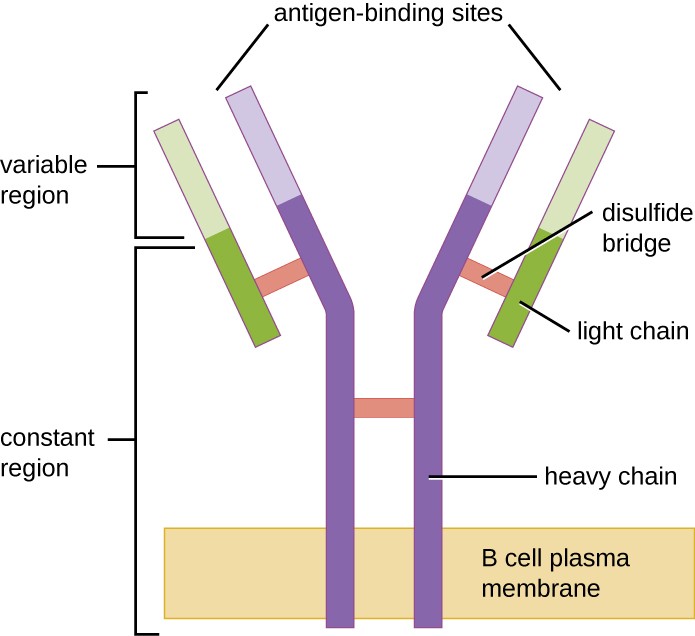
A B cell plasma membrane has two long rectangles spanning it; these form a Y shape. Two shorter rectangles sit on the outside of the upper portion of the Y. The region spanning the membrane and half-way through the bars of the Y is the constant region. The upper region is the variable region which has the antigen binding sites. The long rectangles are the heavy chain. The shorter rectangles are the light chains. Multiple disulfide bridges hold the constant region together.
Check Your Understanding
- What types of molecules serve as the BCR?
- What are the differences between TCRs and BCRs with respect to antigen recognition?
- Which molecule classes are T-dependent antigens and which are T-independent antigens?
T Cell-Independent Activation of B cells
Activation of B cells without the cooperation of helper T cells is referred to as T cell-independent activation and occurs when BCRs interact with T-independent antigens. T-independent antigens (e.g., polysaccharide capsules, lipopolysaccharide) have repetitive epitope units within their structure, and this repetition allows for the cross-linkage of multiple BCRs, providing the first signal for activation (Figure 18.21). Because T cells are not involved, the second signal has to come from other sources, such as interactions of toll-like receptors with PAMPs or interactions with factors from the complement system.
Once a B cell is activated, it undergoes clonal proliferation and daughter cells differentiate into plasma cells. Plasma cells are antibody factories that secrete large quantities of antibodies. After differentiation, the surface BCRs disappear and the plasma cell secretes pentameric IgM molecules that have the same antigen specificity as the BCRs (Figure 18.21).
The T cell-independent response is short-lived and does not result in the production of memory B cells. Thus it will not result in a secondary response to subsequent exposures to T-independent antigens.
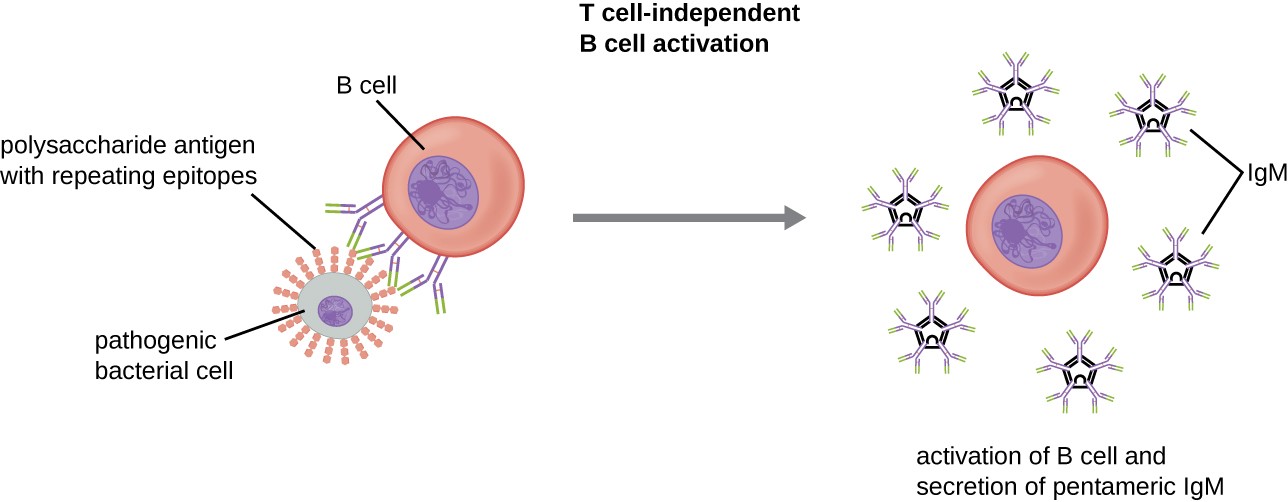
alt=”A circle with small chains of hexagons projecting from the surface is a pathogenic bacterial cell. The chains are polysaccharide antigens with repeating epitopes. Antibodies on the B cell bind to these epitopes. This causes the activation of the B cell and secretion of pentameric IgM.
Check Your Understanding
- What are the two signals required for T cell-independent activation of B cells?
- What is the function of a plasma cell?
T Cell-Dependent Activation of B cells
T cell-dependent activation of B cells is more complex than T cell-independent activation, but the resulting immune response is stronger and develops memory. T cell-dependent activation can occur either in response to free protein antigens or to protein antigens associated with an intact pathogen. Interaction between the BCRs on a naïve mature B cell and a free protein antigen stimulate internalization of the antigen, whereas interaction with antigens associated with an intact pathogen initiates the extraction of the antigen from the pathogen before internalization. Once internalized inside the B cell, the protein antigen is processed and presented with MHC II. The presented antigen is then recognized by helper T cells specific to the same antigen. The TCR of the helper T cell recognizes the foreign antigen, and the T cell’s CD4 molecule interacts with MHC II on the B cell. The coordination between B cells and helper T cells that are specific to the same antigen is referred to as linked recognition.
Once activated by linked recognition, TH2 cells produce and secrete cytokines that activate the B cell and cause proliferation into clonal daughter cells. After several rounds of proliferation, additional cytokines provided by the TH2 cells stimulate the differentiation of activated B cell clones into memory B cells, which will quickly respond to subsequent exposures to the same protein epitope, and plasma cells that lose their membrane BCRs and initially secrete pentameric IgM (Figure 18.22).
After initial secretion of IgM, cytokines secreted by TH2 cells stimulate the plasma cells to switch from IgM production to production of IgG, IgA, or IgE. This process, called class switching or isotype switching, allows plasma cells cloned from the same activated B cell to produce a variety of antibody classes with the same epitope specificity. Class switching is accomplished by genetic rearrangement of gene segments encoding the constant region, which determines an antibody’s class. The variable region is not changed, so the new class of antibody retains the original epitope specificity.
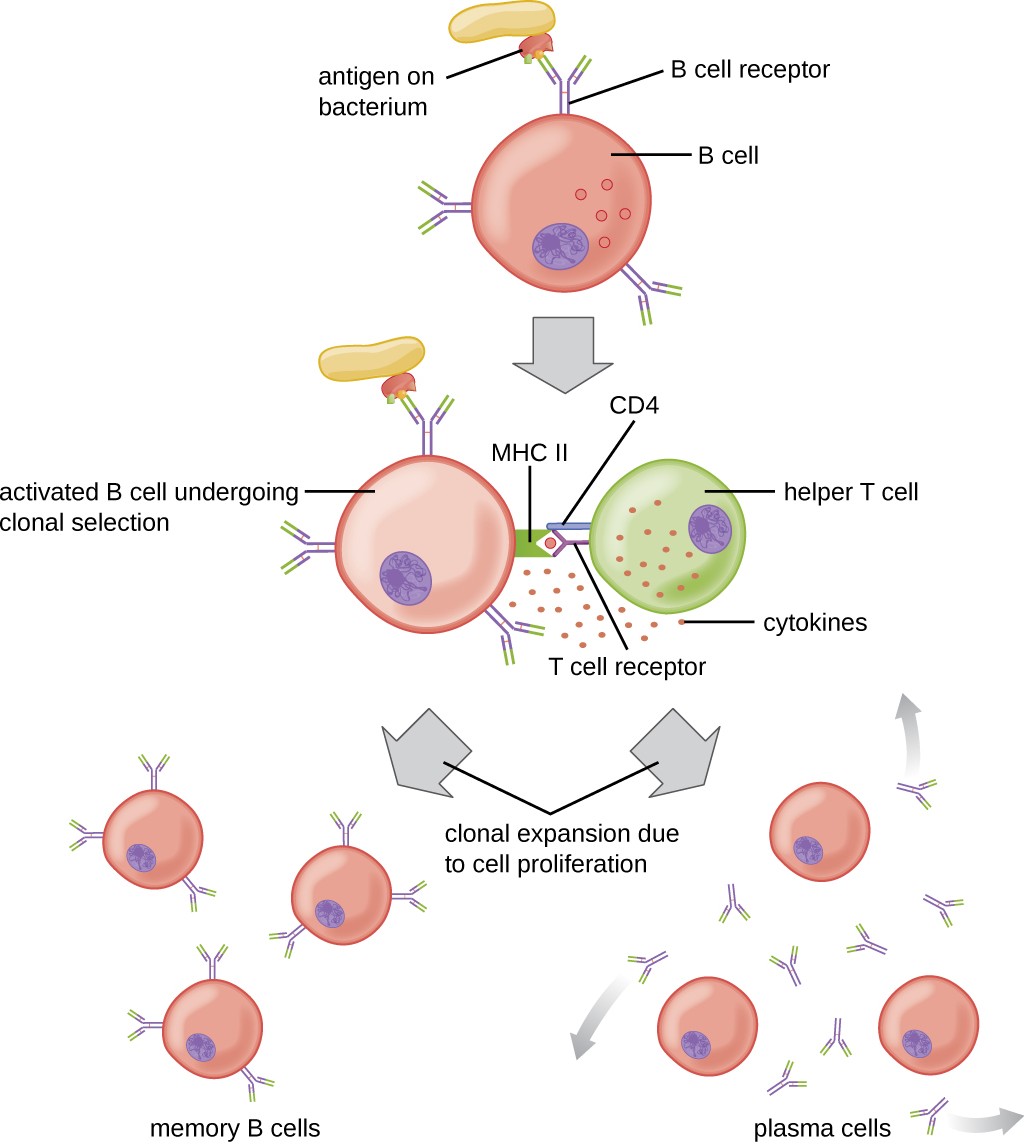
1: BCR interaction with antigen on intact pathogen. An antigen on the surface of a bacterium binds to the B cell receptor on the B cell. s: Antigen processing and presentation with MHC II. The antigen is on the MHC II. 3: Antigen presentation and activation of helper T cell. T cell receptor of helper T cell binds to antigen on MHCII. This is stabilized by CD4. Helper T releases cytokines. 4: Cytokines stimulate clonal proliferation and differentiation into memory B cells and antibody-secreting plasma cells.
Check Your Understanding
- What steps are required for T cell-dependent activation of B cells?
- What is antibody class switching and why is it important?
Primary and Secondary Responses
T cell-dependent activation of B cells plays an important role in both the primary and secondary responses associated with adaptive immunity. With the first exposure to a protein antigen, a T cell-dependent primary antibody response occurs. The initial stage of the primary response is a lag period, or latent period, of approximately 10 days, during which no antibody can be detected in serum. This lag period is the time required for all of the steps of the primary response, including naïve mature B cell binding of antigen with BCRs, antigen processing and presentation, helper T cell activation, B cell activation, and clonal proliferation. The end of the lag period is characterized by a rise in IgM levels in the serum, as TH2 cells stimulate B cell differentiation into plasma cells. IgM levels reach their peak around 14 days after primary antigen exposure; at about this same time, TH2 stimulates antibody class switching, and IgM levels in serum begin to decline. Meanwhile, levels of IgG increase until they reach a peak about three weeks into the primary response (Figure 18.23).
During the primary response, some of the cloned B cells are differentiated into memory B cells programmed to respond to subsequent exposures. This secondary response occurs more quickly and forcefully than the primary response. The lag period is decreased to only a few days and the production of IgG is significantly higher than observed for the primary response (Figure 18.23). In addition, the antibodies produced during the secondary response are more effective and bind with higher affinity to the targeted epitopes. Plasma cells produced during secondary responses live longer than those produced during the primary response, so levels of specific antibody remain elevated for a longer period of time.
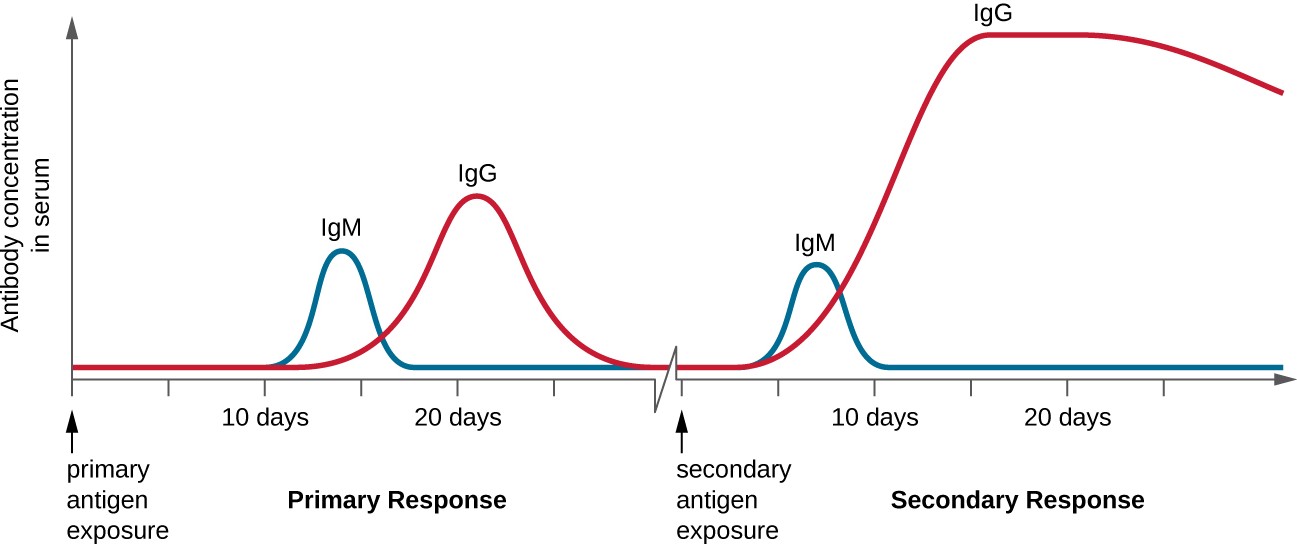
A graph with time on the X axis and antibody concentration in serum. At first there is very little antibody (near 0). The lag period does not see a significant increase. In the primary response, IgM peaks for about 5 days and drops. At the same time IgG increases and then drops. This creates an increase in antibody count with a plateau of about 5 days as both antibody types are present. The secondary response sees a peak of IgM for about 1 to 2 days and then a prolonged peak of IgG. The total antibody is also higher but isn’t at its plateau for as long as it is in the primary response.
Check Your Understanding
- What events occur during the lag period of the primary antibody response?
- Why do antibody levels remain elevated longer during the secondary antibody response?

