3.4 – Unique Characteristics of Eukaryotic Cells
Learning Objectives
- Explain the distinguishing characteristics of eukaryotic cells
- Describe internal and external structures of eukaryotic cells in terms of their physical structure, chemical structure, and function
- Identify and describe structures and organelles unique to eukaryotic cells
- Compare and contrast similar structures found in prokaryotic and eukaryotic cells
Eukaryotic organisms include protozoans, algae, fungi, plants, and animals. Some eukaryotic cells are independent, single-celled microorganisms, whereas others are part of multicellular organisms. The cells of eukaryotic organisms have several distinguishing characteristics. Above all, eukaryotic cells are defined by the presence of a nucleus surrounded by a complex nuclear membrane. Also, eukaryotic cells are characterized by the presence of membrane-bound organelles in the cytoplasm. Organelles such as mitochondria, the endoplasmic reticulum (E.R.), Golgi apparatus, lysosomes, and peroxisomes are held in place by the cytoskeleton, an internal network that supports transport of intracellular components and helps maintain cell shape (Figure 3.35). The genome of eukaryotic cells is packaged in multiple, rod-shaped chromosomes as opposed to the single, circular-shaped chromosome that characterizes most prokaryotic cells. Table 3.2 compares the characteristics of eukaryotic cell structures with those of bacteria and archaea.
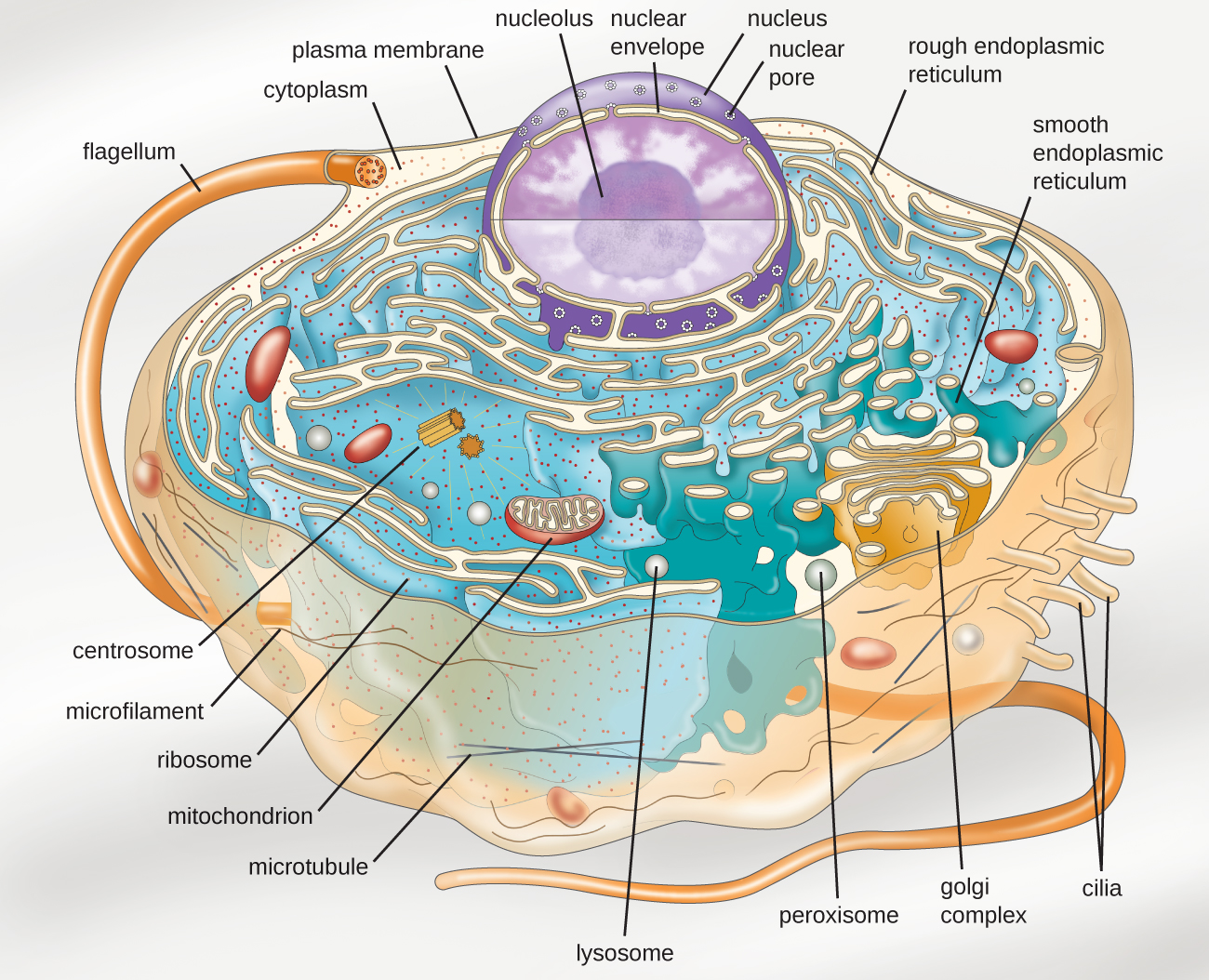
Summary of Cell Structures
| Cell Structure | Prokaryotes(Bacteria) | Prokaryotes(Archaea) | Eukaryotes |
|---|---|---|---|
| Size | ~0.5–1 μM | ~0.5–1 μM | ~5–20 μM |
| Surface area-to-volume ratio | High | High | Low |
| Nucleus | No | No | Yes |
| Genome characteristics |
|
|
|
| Cell division | Binary fission | Binary fission | Mitosis, meiosis |
| Membrane lipid composition |
|
|
|
| Cell wall composition |
|
|
|
| Motility structures | Rigid spiral flagella composed of flagellin | Rigid spiral flagella composed of archaeal flagellins | Flexible flagella and cilia composed of microtubules |
| Membrane-bound organelles | No | No | Yes |
| Endomembrane system | No | No | Yes (E.R., Golgi, lysosomes) |
| Ribosomes | 70 S | 70 S |
|
Cell Morphologies
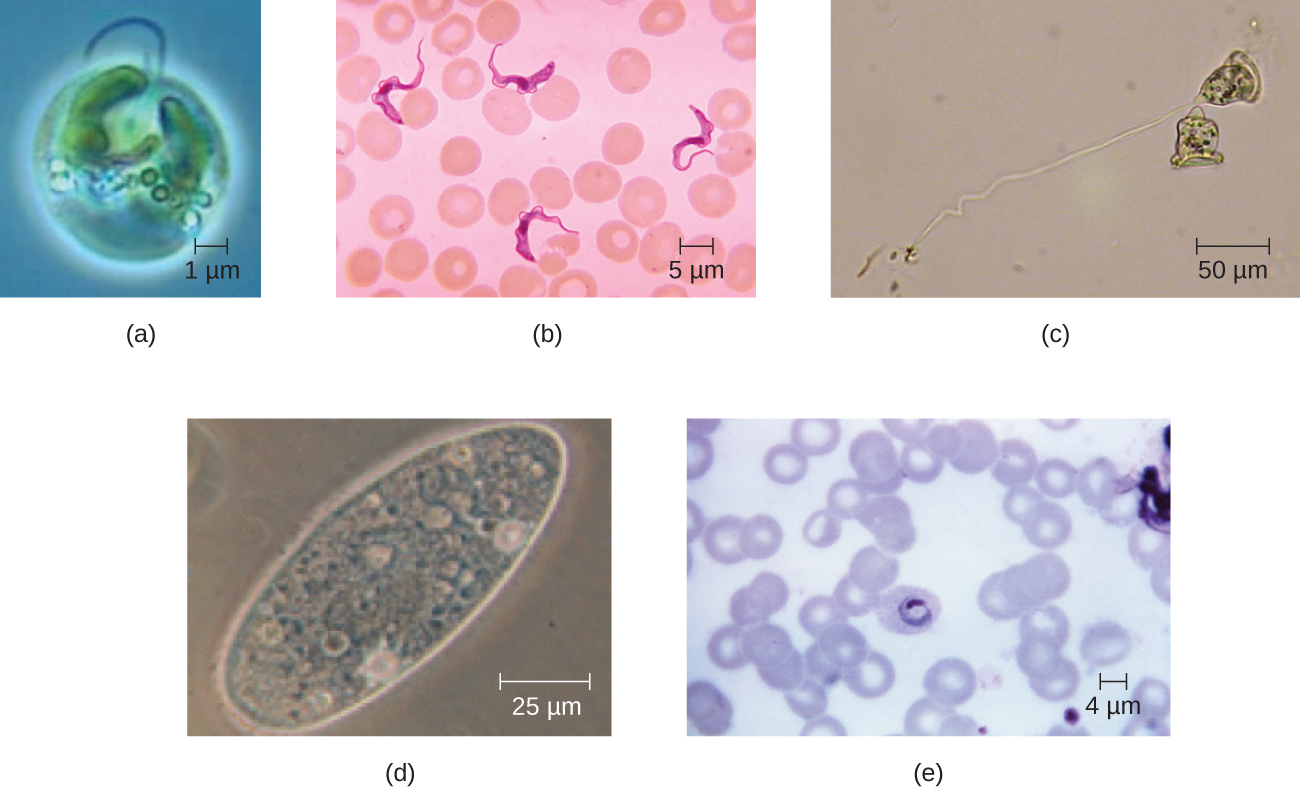
Eukaryotic cells display a wide variety of different cell morphologies. Possible shapes include spheroid, ovoid, cuboidal, cylindrical, flat, lenticular, fusiform, discoidal, crescent, ring stellate, and polygonal (Figure 3.36). Some eukaryotic cells are irregular in shape, and some are capable of changing shape. The shape of a particular type of eukaryotic cell may be influenced by factors such as its primary function, the organization of its cytoskeleton, the viscosity of its cytoplasm, the rigidity of its cell membrane or cell wall (if it has one), and the physical pressure exerted on it by the surrounding environment and/or adjoining cells.
Check Your Understanding
- Identify two differences between eukaryotic and prokaryotic cells
Nucleus
Unlike prokaryotic cells, in which D.N.A. is loosely contained in the nucleoid region, eukaryotic cells possess a nucleus, which is surrounded by a complex nuclear membrane that houses the D.N.A. genome (Figure 3.37). By containing the cell’s D.N.A., the nucleus ultimately controls all activities of the cell and also serves an essential role in reproduction and heredity. Eukaryotic cells typically have their D.N.A. organized into multiple linear chromosomes. The D.N.A. within the nucleus is highly organized and condensed to fit inside the nucleus, which is accomplished by wrapping the D.N.A. around proteins called histones.
Although most eukaryotic cells have only one nucleus, exceptions exist. For example, protozoans of the genus Paramecium typically have two complete nuclei: a small nucleus that is used for reproduction (micronucleus) and a large nucleus that directs cellular metabolism (macronucleus). Additionally, some fungi transiently form cells with two nuclei, called heterokaryotic cells, during sexual reproduction. Cells whose nuclei divide, but whose cytoplasm does not, are called coenocytes.
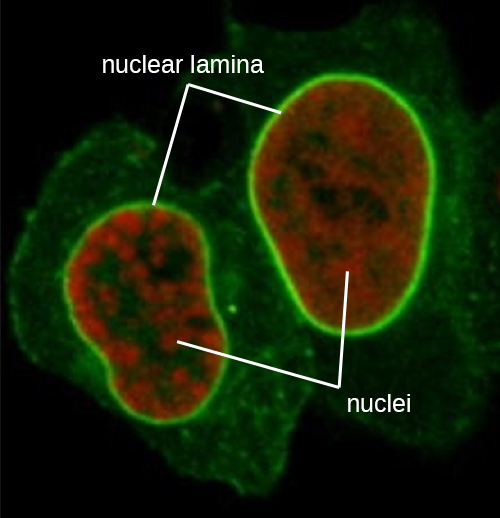
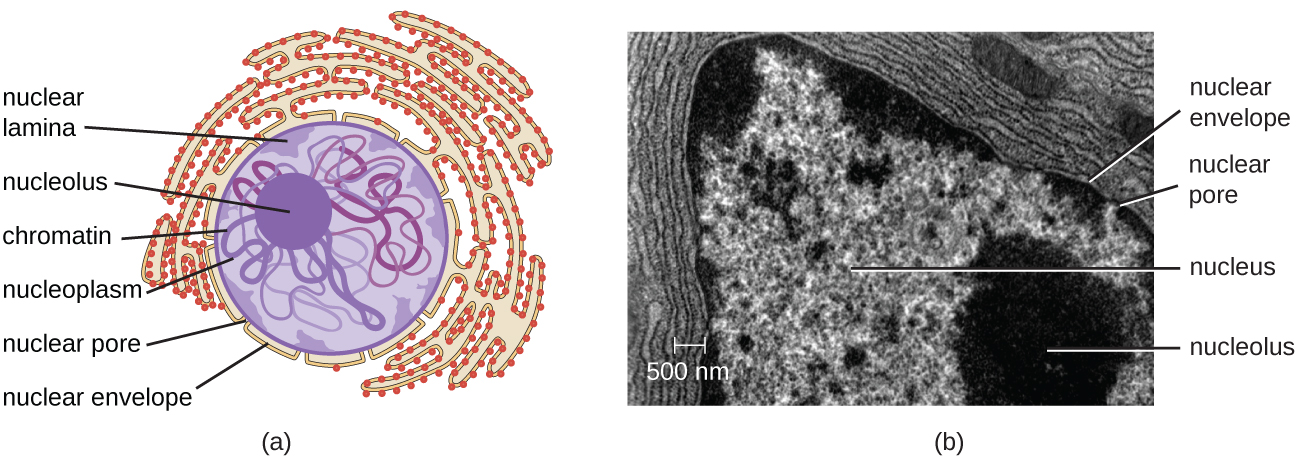
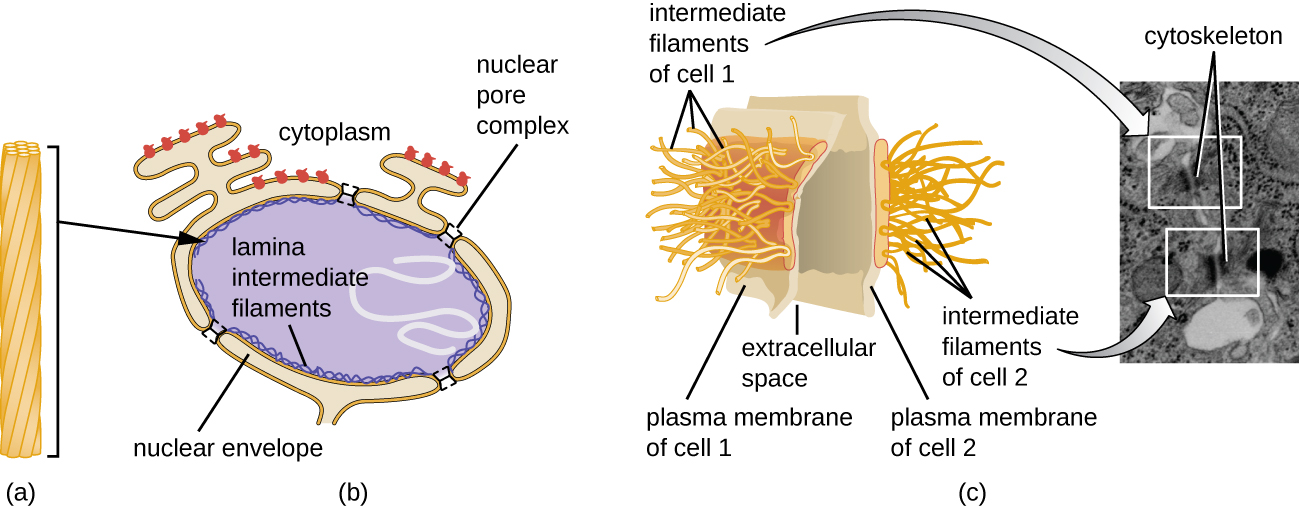
The nucleus is bound by a complex nuclear membrane, often called the nuclear envelope, that consists of two distinct lipid bilayers that are contiguous with each other (Figure 3.38). Despite these connections between the inner and outer membranes, each membrane contains unique lipids and proteins on its inner and outer surfaces. The nuclear envelope contains nuclear pores, which are large, rosette-shaped protein complexes that control the movement of materials into and out of the nucleus. The overall shape of the nucleus is determined by the nuclear lamina, a meshwork of intermediate filaments found just inside the nuclear envelope membranes. Outside the nucleus, additional intermediate filaments form a looser mesh and serve to anchor the nucleus in position within the cell.
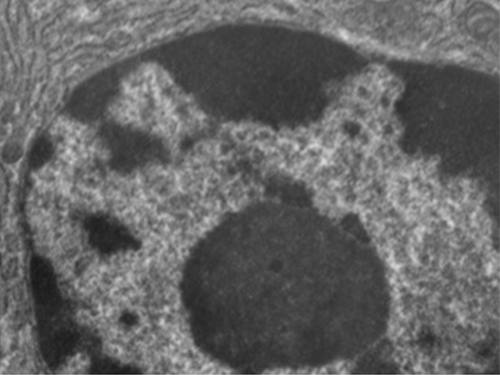
Nucleolus
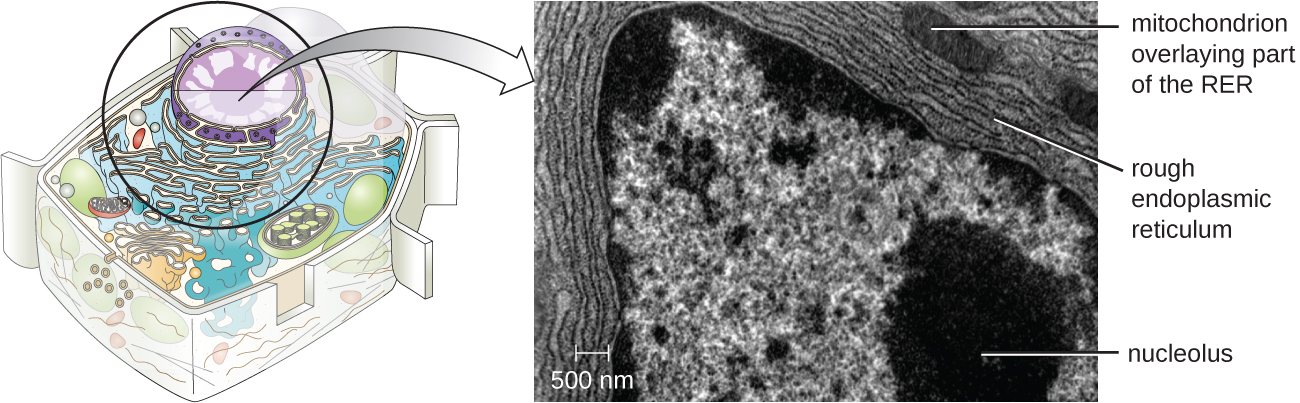
The nucleolus is a dense region within the nucleus where ribosomal R.N.A. (r R.N.A.) biosynthesis occurs. In addition, the nucleolus is also the site where assembly of ribosomes begins. Preribosomal complexes are assembled from r R.N.A. and proteins in the nucleolus; they are then transported out to the cytoplasm, where ribosome assembly is completed (Figure 3.39)
Ribosomes
Ribosomes found in eukaryotic organelles such as mitochondria or chloroplasts have 70 S ribosomes—the same size as prokaryotic ribosomes. However, nonorganelle-associated ribosomes in eukaryotic cells are 80 S ribosomes, composed of a 40S small subunit and a 60 S large subunit. In terms of size and composition, this makes them distinct from the ribosomes of prokaryotic cells.
The two types of nonorganelle-associated eukaryotic ribosomes are defined by their location in the cell: free ribosomes and membrane-bound ribosomes. Free ribosomes are found in the cytoplasm and serve to synthesize water-soluble proteins; membrane-bound ribosomes are found attached to the rough endoplasmic reticulum and make proteins for insertion into the cell membrane or proteins destined for export from the cell.
The differences between eukaryotic and prokaryotic ribosomes are clinically relevant because certain antibiotic drugs are designed to target one or the other. For example, cycloheximide targets eukaryotic action, whereas chloramphenicol targets prokaryotic ribosomes.24 Since human cells are eukaryotic, they generally are not harmed by antibiotics that destroy the prokaryotic ribosomes in bacteria. However, sometimes negative side effects may occur because mitochondria in human cells contain prokaryotic ribosomes.
Endomembrane System
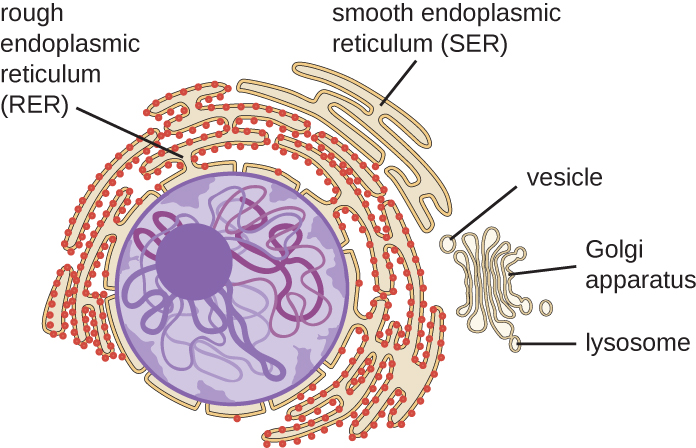
The endomembrane system, unique to eukaryotic cells, is a series of membranous tubules, sacs, and flattened disks that synthesize many cell components and move materials around within the cell (Figure 3.40). Because of their larger cell size, eukaryotic cells require this system to transport materials that cannot be dispersed by diffusion alone. The endomembrane system comprises several organelles and connections between them, including the endoplasmic reticulum, Golgi apparatus, lysosomes, and vesicles.
Endoplasmic Reticulum
The endoplasmic reticulum (E.R.) is an interconnected array of tubules and cisternae (flattened sacs) with a single lipid bilayer (Figure 3.41). The spaces inside of the cisternae are called lumen of the E.R. There are two types of E.R., rough endoplasmic reticulum (R.E.R.) and smooth endoplasmic reticulum (S.E.R.). These two different types of E.R. are sites for the synthesis of distinctly different types of molecules. R.E.R is studded with ribosomes bound on the cytoplasmic side of the membrane. These ribosomes make proteins destined for the plasma membrane (Figure 3.41). Following synthesis, these proteins are inserted into the membrane of the R.E.R. Small sacs of the R.E.R. containing these newly synthesized proteins then bud off as transport vesicles and move either to the Golgi apparatus for further processing, directly to the plasma membrane, to the membrane of another organelle, or out of the cell. Transport vesicles are single-lipid, bilayer, membranous spheres with hollow interiors that carry molecules. S.E.R. does not have ribosomes and, therefore, appears “smooth.” It is involved in biosynthesis of lipids, carbohydrate metabolism, and detoxification of toxic compounds within the cell.
Golgi Apparatus
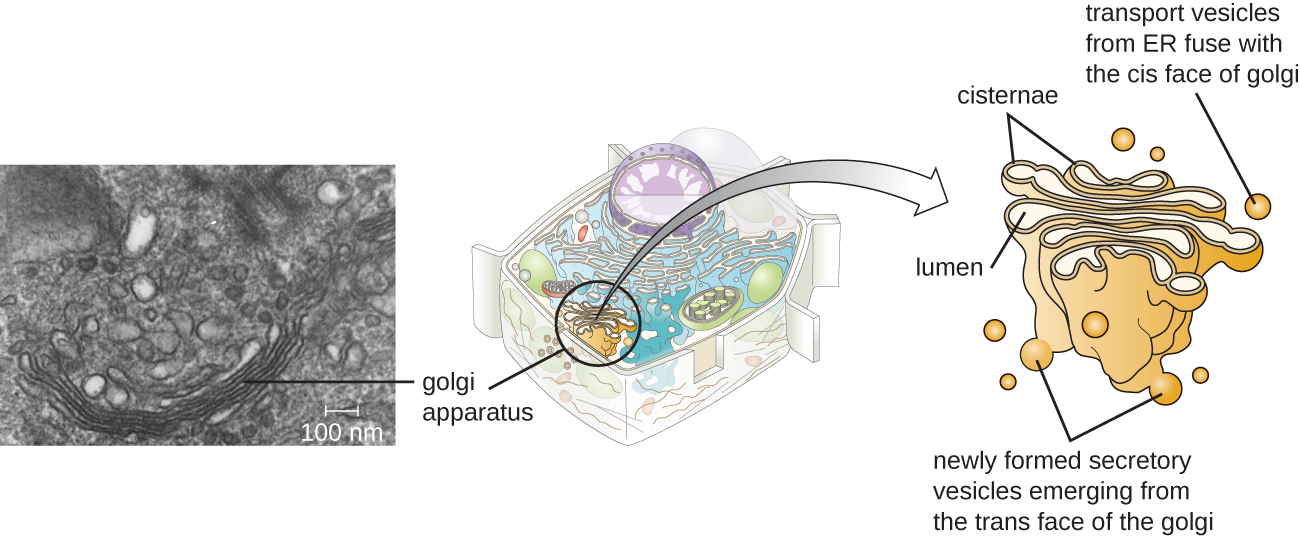
The Golgi apparatus was discovered within the endomembrane system in 1898 by Italian scientist Camillo Golgi (1843–1926), who developed a novel staining technique that showed stacked membrane structures within the cells of Plasmodium, the causative agent of malaria. The Golgi apparatus is composed of a series of membranous disks called dictyosomes, each having a single lipid bilayer, that are stacked together (Figure 3.42).
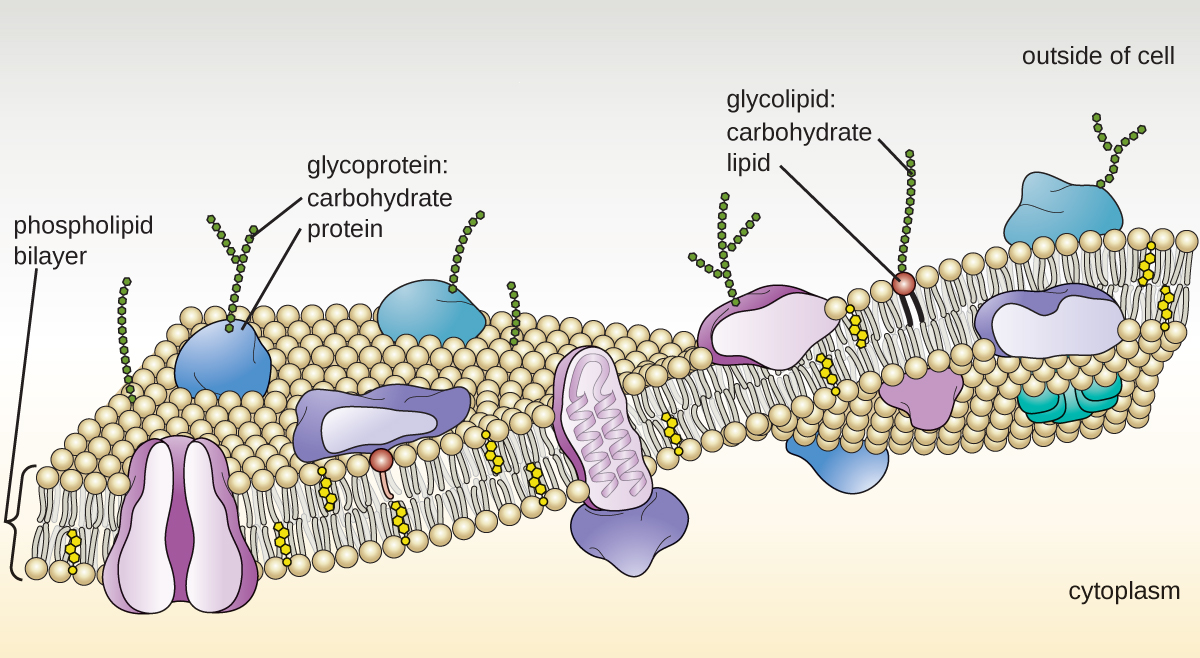
Enzymes in the Golgi apparatus modify lipids and proteins transported from the E.R. to the Golgi, often adding carbohydrate components to them, producing glycolipids, glycoproteins, or proteoglycans. Glycolipids and glycoproteins are often inserted into the plasma membrane and are important for signal recognition by other cells or infectious particles. Different types of cells can be distinguished from one another by the structure and arrangement of the glycolipids and glycoproteins contained in their plasma membranes. These glycolipids and glycoproteins commonly also serve as cell surface receptors.
Transport vesicles leaving the E.R. fuse with a Golgi apparatus on its receiving, or cis, face. The proteins are processed within the Golgi apparatus, and then additional transport vesicles containing the modified proteins and lipids pinch off from the Golgi apparatus on its outgoing, or trans, face. These outgoing vesicles move to and fuse with the plasma membrane or the membrane of other organelles.
Exocytosis is the process by which secretory vesicles (spherical membranous sacs) release their contents to the cell’s exterior (Figure 3.42). All cells have constitutive secretory pathways in which secretory vesicles transport soluble proteins that are released from the cell continually (constitutively). Certain specialized cells also have regulated secretory pathways, which are used to store soluble proteins in secretory vesicles. Regulated secretion involves substances that are only released in response to certain events or signals. For example, certain cells of the human immune system (e.g., mast cells) secrete histamine in response to the presence of foreign objects or pathogens in the body. Histamine is a compound that triggers various mechanisms used by the immune system to eliminate pathogens.
Lysosomes
In the 1960s, Belgian scientist Christian de Duve (1917–2013) discovered lysosomes, membrane-bound organelles of the endomembrane system that contain digestive enzymes. Certain types of eukaryotic cells use lysosomes to break down various particles, such as food, damaged organelles or cellular debris, microorganisms, or immune complexes. Compartmentalization of the digestive enzymes within the lysosome allows the cell to efficiently digest matter without harming the cytoplasmic components of the cell.
Check Your Understanding
- Name the components of the endomembrane system and describe the function of each component.
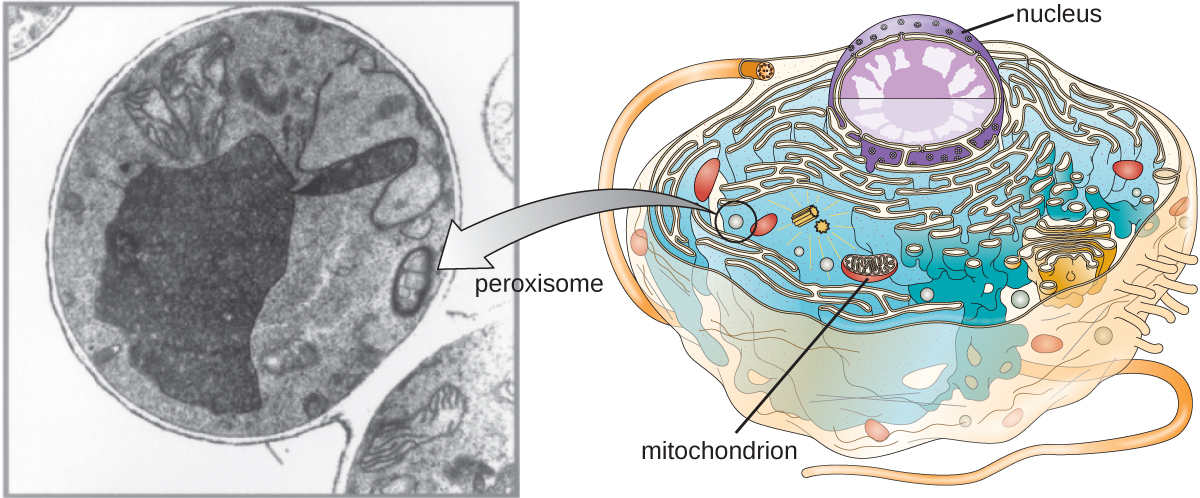
Peroxisomes
Christian de Duve is also credited with the discovery of peroxisomes, membrane-bound organelles that are not part of the endomembrane system (Figure 3.43). Peroxisomes form independently in the cytoplasm from the synthesis of peroxin proteins by free ribosomes and the incorporation of these peroxin proteins into existing peroxisomes. Growing peroxisomes then divide by a process similar to binary fission.
Peroxisomes were first named for their ability to produce hydrogen peroxide, a highly reactive molecule that helps to break down molecules such as uric acid, amino acids, and fatty acids. Peroxisomes also possess the enzyme catalase, which can degrade hydrogen peroxide. Along with the S.E.R., peroxisomes also play a role in lipid biosynthesis. Like lysosomes, the compartmentalization of these degradative molecules within an organelle helps protect the cytoplasmic contents from unwanted damage.
The peroxisomes of certain organisms are specialized to meet their particular functional needs. For example, glyoxysomes are modified peroxisomes of yeasts and plant cells that perform several metabolic functions, including the production of sugar molecules. Similarly, glycosomes are modified peroxisomes made by certain trypanosomes, the pathogenic protozoans that cause Chagas disease and African sleeping sickness.
Cytoskeleton
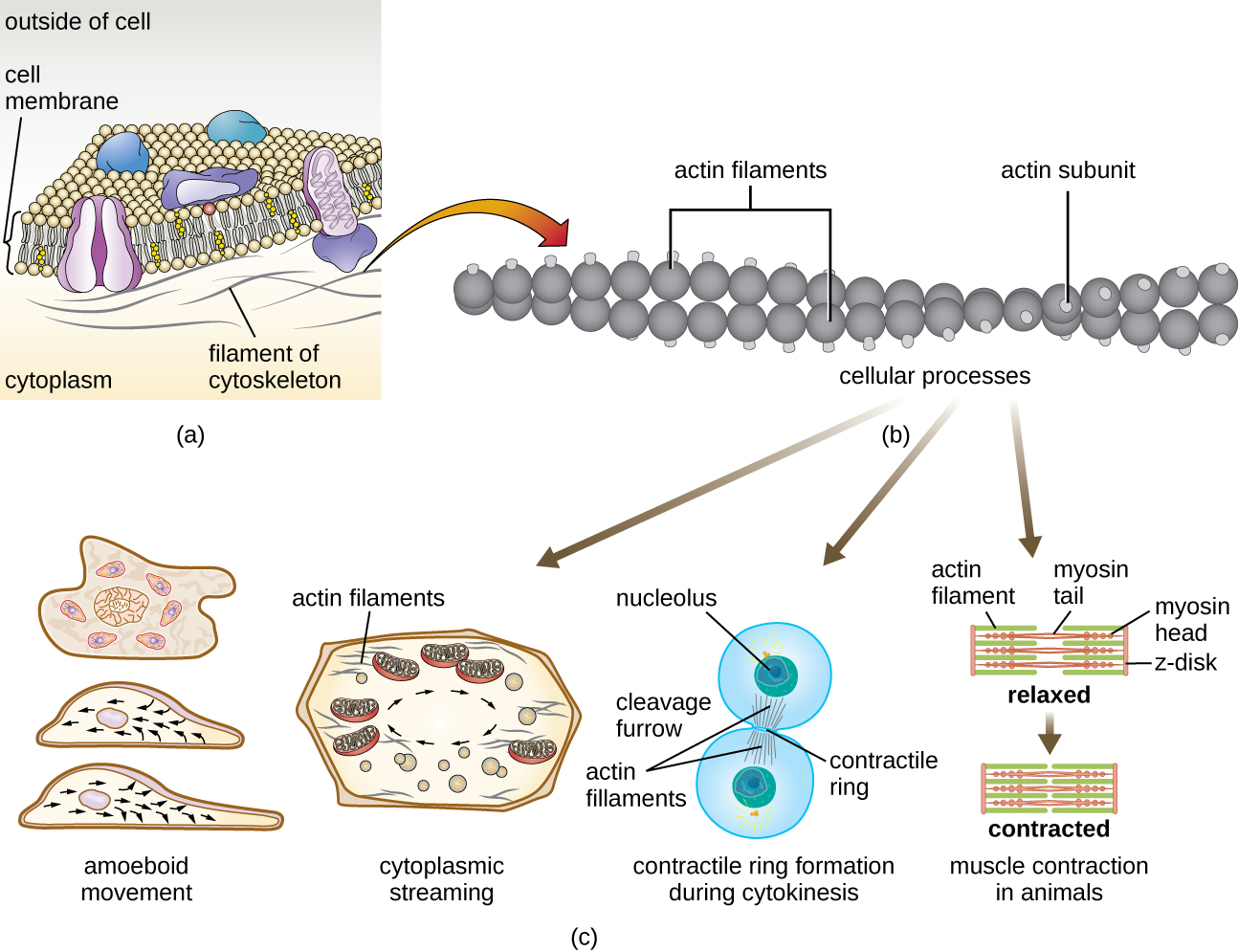
Eukaryotic cells have an internal cytoskeleton made of microfilaments, intermediate filaments, and microtubules. This matrix of fibers and tubes provides structural support as well as a network over which materials can be transported within the cell and on which organelles can be anchored (Figure 3.44). For example, the process of exocytosis involves the movement of a vesicle via the cytoskeletal network to the plasma membrane, where it can release its contents.
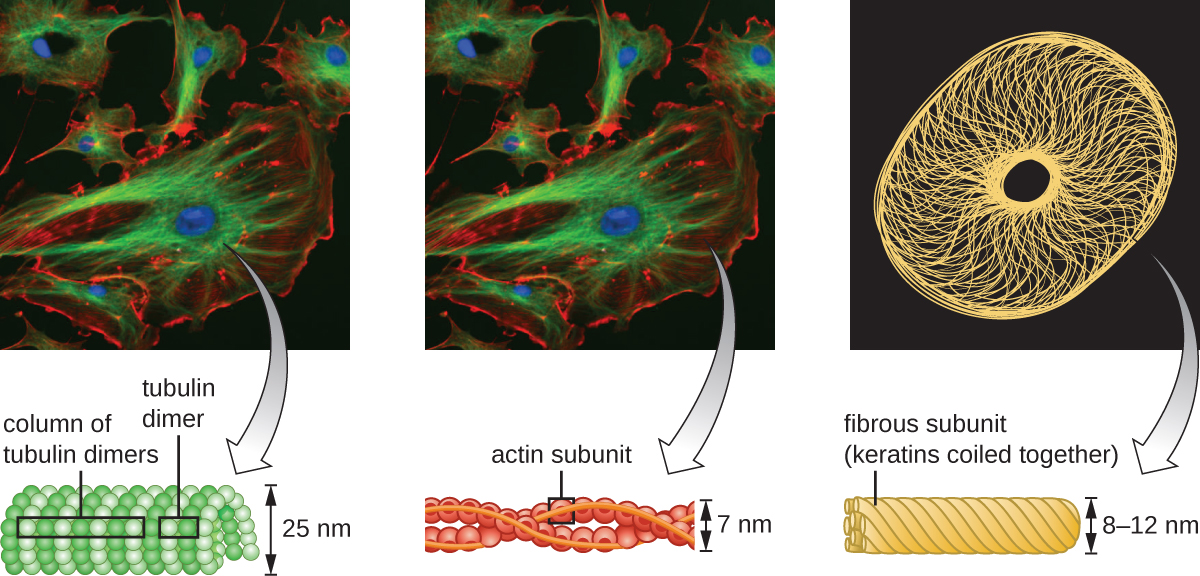
Microfilaments are composed of two intertwined strands of actin, each composed of actin monomers forming filamentous cables 6 nm in diameter25 (Figure 3.45). The actin filaments work together with motor proteins, like myosin, to effect muscle contraction in animals or the amoeboid movement of some eukaryotic microbes. In ameboid organisms, actin can be found in two forms: a stiffer, polymerized, gel form and a more fluid, unpolymerized soluble form. Actin in the gel form creates stability in the ectoplasm, the gel-like area of cytoplasm just inside the plasma membrane of ameboid protozoans.
Temporary extensions of the cytoplasmic membrane called pseudopodia (meaning “false feet”) are produced through the forward flow of soluble actin filaments into the pseudopodia, followed by the gel-sol cycling of the actin filaments, resulting in cell motility. Once the cytoplasm extends outward, forming a pseudopodium, the remaining cytoplasm flows up to join the leading edge, thereby creating forward locomotion. Beyond amoeboid movement, microfilaments are also involved in a variety of other processes in eukaryotic cells, including cytoplasmic streaming (the movement or circulation of cytoplasm within the cell), cleavage furrow formation during cell division, and muscle movement in animals (Figure 3.45). These functions are the result of the dynamic nature of microfilaments, which can polymerize and depolymerize relatively easily in response to cellular signals, and their interactions with molecular motors in different types of eukaryotic cells.
Intermediate filaments (Figure 3.46) are a diverse group of cytoskeletal filaments that act as cables within the cell. They are termed “intermediate” because their 10-nm diameter is thicker than that of actin but thinner than that of microtubules.26 They are composed of several strands of polymerized subunits that, in turn, are made up of a wide variety of monomers. Intermediate filaments tend to be more permanent in the cell and maintain the position of the nucleus. They also form the nuclear lamina (lining or layer) just inside the nuclear envelope. Additionally, intermediate filaments play a role in anchoring cells together in animal tissues. The intermediate filament protein desmin is found in desmosomes, the protein structures that join muscle cells together and help them resist external physical forces. The intermediate filament protein keratin is a structural protein found in hair, skin, and nails.
Microtubules (Figure 3.47) are a third type of cytoskeletal fiber composed of tubulin dimers (α tubulin and β tubulin). These form hollow tubes 23 nm in diameter that are used as girders within the cytoskeleton.27 Like microfilaments, microtubules are dynamic and have the ability to rapidly assemble and disassemble. Microtubules also work with motor proteins (such as dynein and kinesin) to move organelles and vesicles around within the cytoplasm. Additionally, microtubules are the main components of eukaryotic flagella and cilia, composing both the filament and the basal body components (Figure 3.54).
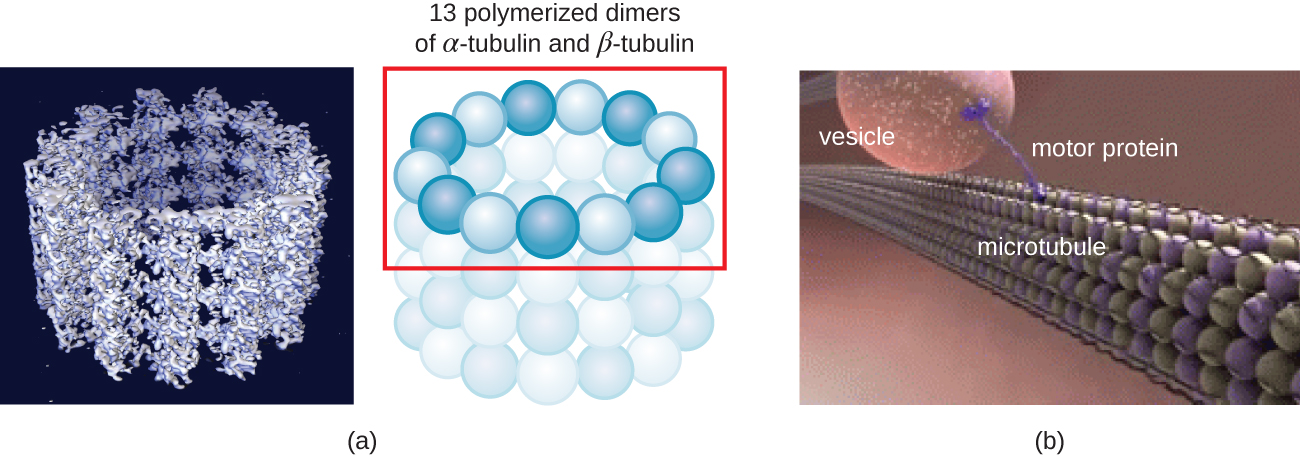
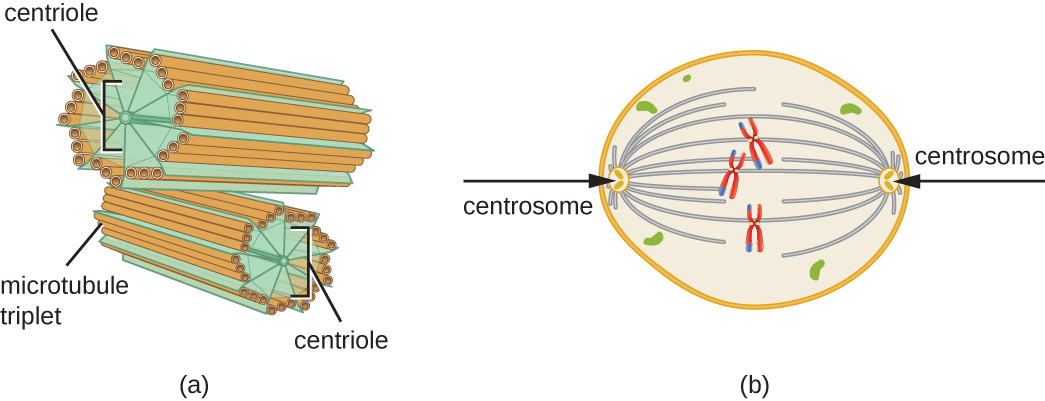
In addition, microtubules are involved in cell division, forming the mitotic spindle that serves to separate chromosomes during mitosis and meiosis. The mitotic spindle is produced by two centrosomes, which are essentially microtubule-organizing centers, at opposite ends of the cell. Each centrosome is composed of a pair of centrioles positioned at right angles to each other, and each centriole is an array of nine parallel microtubules arranged in triplets (Figure 3.48).
Check Your Understanding
- Compare and contrast the three types of cytoskeletal structures described in this section.
Mitochondria
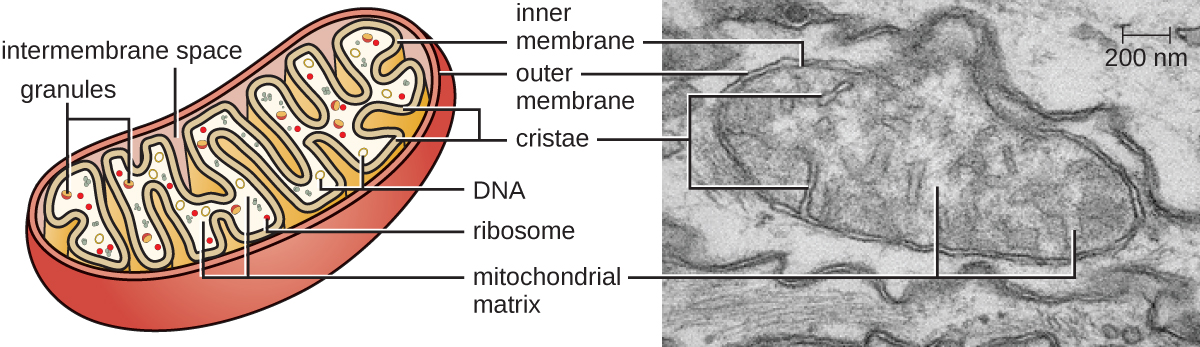
The large, complex organelles in which aerobic cellular respiration occurs in eukaryotic cells are called mitochondria (Figure 3.49). The term “mitochondrion” was first coined by German microbiologist Carl Benda in 1898 and was later connected with the process of respiration by Otto Warburg in 1913. Scientists during the 1960s discovered that mitochondria have their own genome and 70 S ribosomes. The mitochondrial genome was found to be bacterial, when it was sequenced in 1976. These findings ultimately supported the endosymbiotic theory proposed by Lynn Margulis, which states that mitochondria originally arose through an endosymbiotic event in which a bacterium capable of aerobic cellular respiration was taken up by phagocytosis into a host cell and remained as a viable intracellular component.
Each mitochondrion has two lipid membranes. The outer membrane is a remnant of the original host cell’s membrane structures. The inner membrane was derived from the bacterial plasma membrane. The electron transport chain for aerobic respiration uses integral proteins embedded in the inner membrane. The mitochondrial matrix, corresponding to the location of the original bacterium’s cytoplasm, is the current location of many metabolic enzymes. It also contains mitochondrial D.N.A. and 70 S ribosomes. Invaginations of the inner membrane, called cristae, evolved to increase surface area for the location of biochemical reactions. The folding patterns of the cristae differ among various types of eukaryotic cells and are used to distinguish different eukaryotic organisms from each other.
Chloroplasts
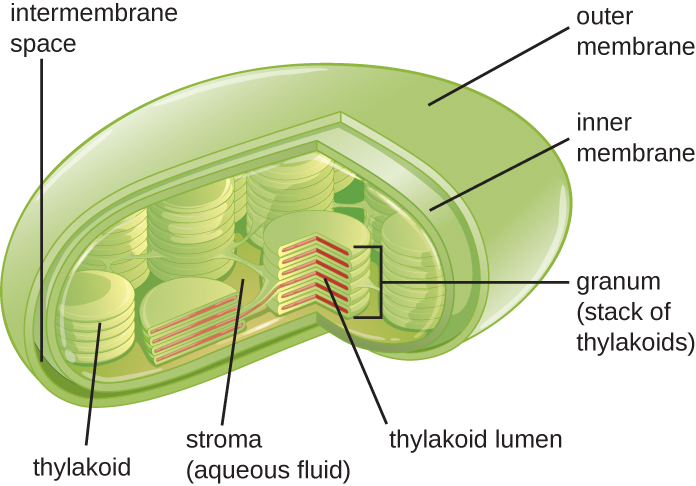
Plant cells and algal cells contain chloroplasts, the organelles in which photosynthesis occurs (Figure 3.50). All chloroplasts have at least three membrane systems: the outer membrane, the inner membrane, and the thylakoid membrane system. Inside the outer and inner membranes is the chloroplast stroma, a gel-like fluid that makes up much of a chloroplast’s volume, and in which the thylakoid system floats. The thylakoid system is a highly dynamic collection of folded membrane sacs. It is where the green photosynthetic pigment chlorophyll is found and the light reactions of photosynthesis occur. In most plant chloroplasts, the thylakoids are arranged in stacks called grana (singular: granum), whereas in some algal chloroplasts, the thylakoids are free floating.
Other organelles similar to mitochondria have arisen in other types of eukaryotes, but their roles differ. Hydrogenosomes are found in some anaerobic eukaryotes and serve as the location of anaerobic hydrogen production. Hydrogenosomes typically lack their own D.N.A. and ribosomes. Kinetoplasts are a variation of the mitochondria found in some eukaryotic pathogens. In these organisms, each cell has a single, long, branched mitochondrion in which kinetoplast D.N.A., organized as multiple circular pieces of D.N.A., is found concentrated at one pole of the cell.
Plasma Membrane
The plasma membrane of eukaryotic cells is similar in structure to the prokaryotic plasma membrane in that it is composed mainly of phospholipids forming a bilayer with embedded peripheral and integral proteins (Figure 3.51). These membrane components move within the plane of the membrane according to the fluid mosaic model. However, unlike the prokaryotic membrane, eukaryotic membranes contain sterols, including cholesterol, that alter membrane fluidity. Additionally, many eukaryotic cells contain some specialized lipids, including sphingolipids, which are thought to play a role in maintaining membrane stability as well as being involved in signal transduction pathways and cell-to-cell communication.
Membrane Transport Mechanisms
The processes of simple diffusion, facilitated diffusion, and active transport are used in both eukaryotic and prokaryotic cells. However, eukaryotic cells also have the unique ability to perform various types of endocytosis, the uptake of matter through plasma membrane invagination and vacuole/vesicle formation (Figure 3.52). A type of endocytosis involving the engulfment of large particles through membrane invagination is called phagocytosis, which means “cell eating.” In phagocytosis, particles (or other cells) are enclosed in a pocket within the membrane, which then pinches off from the membrane to form a vacuole that completely surrounds the particle. Another type of endocytosis is called pinocytosis, which means “cell drinking.” In pinocytosis, small, dissolved materials and liquids are taken into the cell through small vesicles. Saprophytic fungi, for example, obtain their nutrients from dead and decaying matter largely through pinocytosis.
Receptor-mediated endocytosis is a type of endocytosis that is initiated by specific molecules called ligands when they bind to cell surface receptors on the membrane. Receptor-mediated endocytosis is the mechanism that peptide and amine-derived hormones use to enter cells and is also used by various viruses and bacteria for entry into host cells.
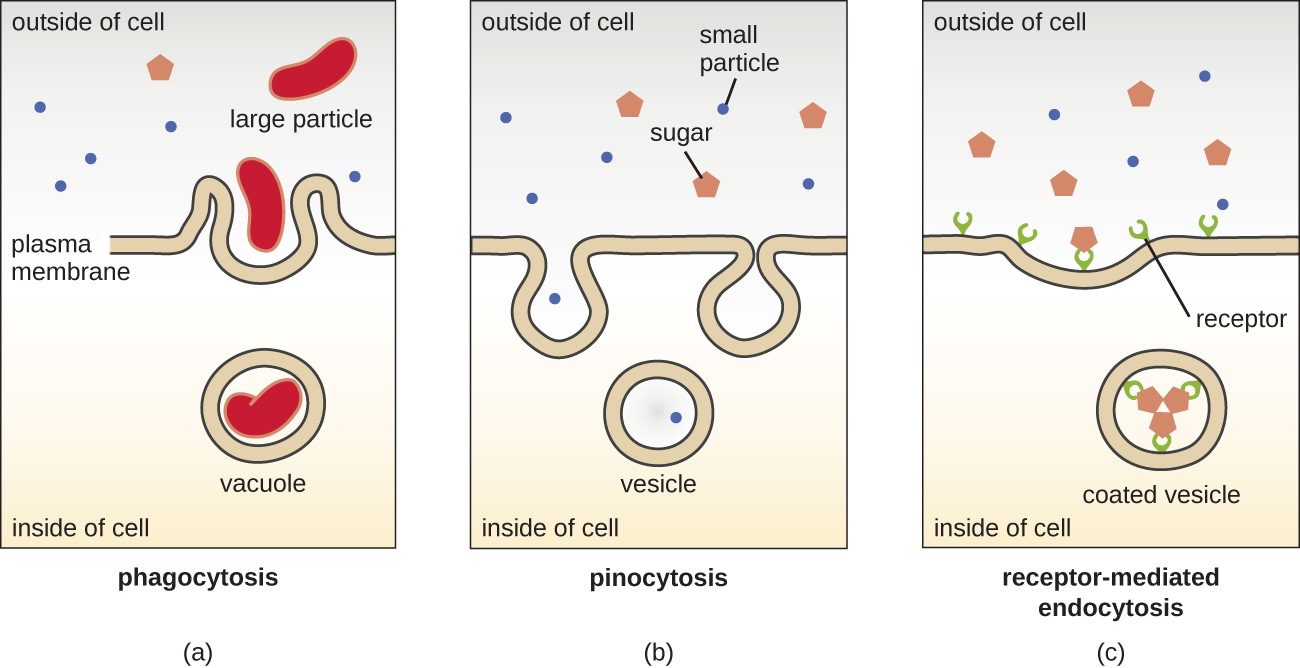
The process by which secretory vesicles release their contents to the cell’s exterior is called exocytosis. Vesicles move toward the plasma membrane and then meld with the membrane, ejecting their contents out of the cell. Exocytosis is used by cells to remove waste products and may also be used to release chemical signals that can be taken up by other cells.
Cell Wall
In addition to a plasma membrane, some eukaryotic cells have a cell wall. Cells of fungi, algae, plants, and even some protists have cell walls. Depending upon the type of eukaryotic cell, cell walls can be made of a wide range of materials, including cellulose (fungi and plants); biogenic silica, calcium carbonate, agar, and carrageenan (protists and algae); or chitin (fungi). In general, all cell walls provide structural stability for the cell and protection from environmental stresses such as desiccation, changes in osmotic pressure, and traumatic injury.29
Extracellular Matrix
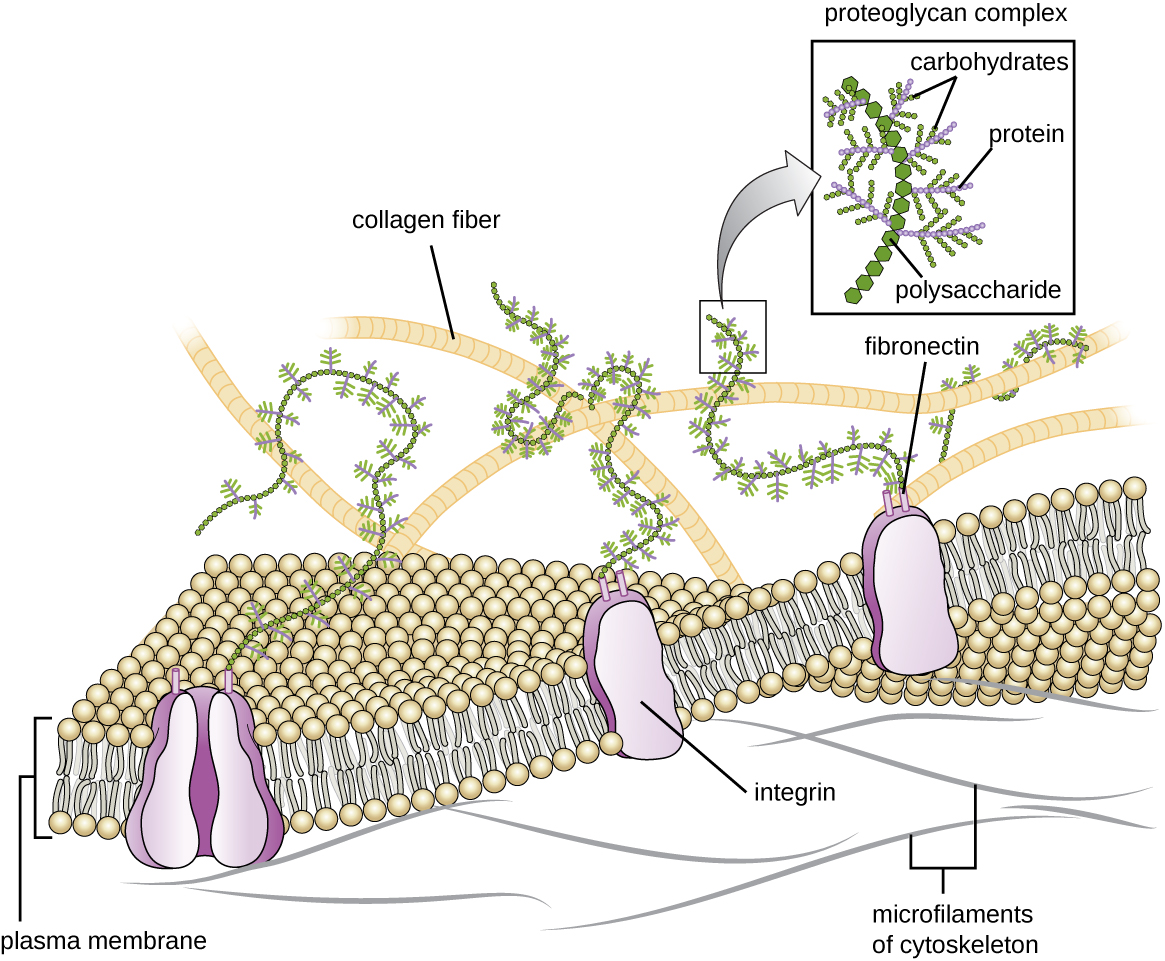
Cells of animals and some protozoans do not have cell walls to help maintain shape and provide structural stability. Instead, these types of eukaryotic cells produce an extracellular matrix for this purpose. They secrete a sticky mass of carbohydrates and proteins into the spaces between adjacent cells (Figure 3.53). Some protein components assemble into a basement membrane to which the remaining extracellular matrix components adhere. Proteoglycans typically form the bulky mass of the extracellular matrix while fibrous proteins, like collagen, provide strength. Both proteoglycans and collagen are attached to fibronectin proteins, which, in turn, are attached to integrin proteins. These integrin proteins interact with transmembrane proteins in the plasma membranes of eukaryotic cells that lack cell walls.
In animal cells, the extracellular matrix allows cells within tissues to withstand external stresses and transmits signals from the outside of the cell to the inside. The amount of extracellular matrix is quite extensive in various types of connective tissues, and variations in the extracellular matrix can give different types of tissues their distinct properties. In addition, a host cell’s extracellular matrix is often the site where microbial pathogens attach themselves to establish infection. For example, Streptococcus pyogenes, the bacterium that causes strep throat and various other infections, binds to fibronectin in the extracellular matrix of the cells lining the oropharynx (upper region of the throat).
Flagella and Cilia
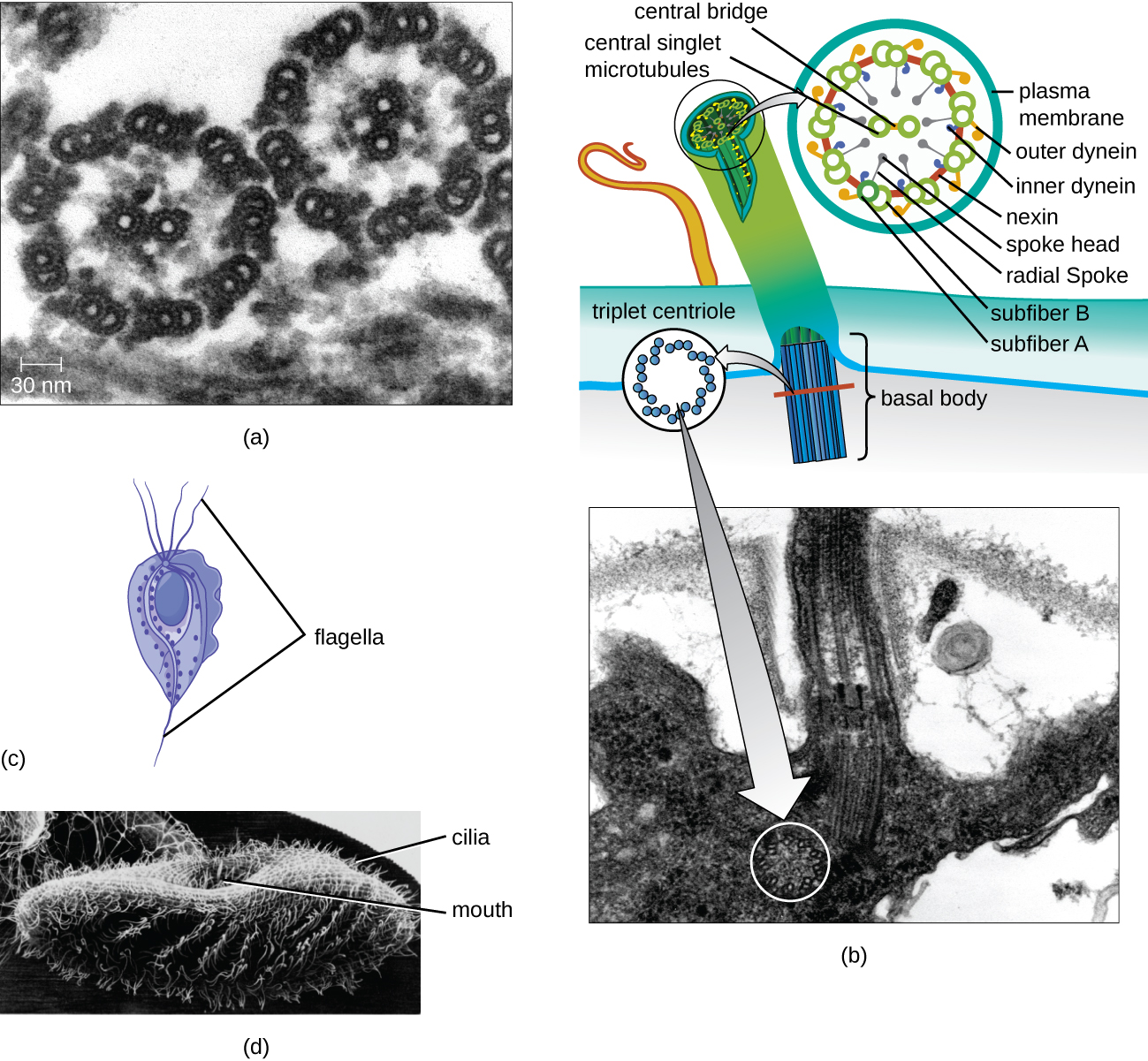
Some eukaryotic cells use flagella for locomotion; however, eukaryotic flagella are structurally distinct from those found in prokaryotic cells. Whereas the prokaryotic flagellum is a stiff, rotating structure, a eukaryotic flagellum is more like a flexible whip composed of nine parallel pairs of microtubules surrounding a central pair of microtubules. This arrangement is referred to as a 9+2 array (Figure 3.54). The parallel microtubules use dynein motor proteins to move relative to each other, causing the flagellum to bend.
Cilia (singular: cilium) are a similar external structure found in some eukaryotic cells. Unique to eukaryotes, cilia are shorter than flagella and often cover the entire surface of a cell; however, they are structurally similar to flagella (a 9+2 array of microtubules) and use the same mechanism for movement. A structure called a basal body is found at the base of each cilium and flagellum. The basal body, which attaches the cilium or flagellum to the cell, is composed of an array of triplet microtubules similar to that of a centriole but embedded in the plasma membrane. Because of their shorter length, cilia use a rapid, flexible, waving motion. In addition to motility, cilia may have other functions such as sweeping particles past or into cells. For example, ciliated protozoans use the sweeping of cilia to move food particles into their mouthparts, and ciliated cells in the mammalian respiratory tract beat in synchrony to sweep mucus and debris up and out of the lungs (Figure 3.54).
Check Your Understanding
- Explain how the cellular envelope of eukaryotic cells compares to that of prokaryotic cells.
- Explain the difference between eukaryotic and prokaryotic flagella.
Footnotes
A.E. Barnhill, M.T. Brewer, S.A. Carlson. “Adverse Effects of Antimicrobials via Predictable or Idiosyncratic Inhibition of Host Mitochondrial Components.” Antimicrobial Agents and Chemotherapy 56 no. 8 (2012):4046–4051.
Fuchs E, Cleveland DW. “A Structural Scaffolding of Intermediate Filaments in Health and Disease.” Science 279 no. 5350 (1998):514–519.
E. Fuchs, D.W. Cleveland. “A Structural Scaffolding of Intermediate Filaments in Health and Disease.” Science 279 no. 5350 (1998):514–519.
E. Fuchs, D.W. Cleveland. “A Structural Scaffolding of Intermediate Filaments in Health and Disease.” Science 279 no. 5350 (1998):514–519.
M. Dudzick. “Protists.” OpenStax CNX. November 27, 2013. http://cnx.org/contents/f7048bb6-e462-459b-805c-ef291cf7049c@1

