12.4 – Gene Therapy
Learning Objectives
- Summarize the mechanisms, risks, and potential benefits of gene therapy
- Identify ethical issues involving gene therapy and the regulatory agencies that provide oversight for clinical trials
- Compare somatic-cell and germ-line gene therapy
Many types of genetic engineering have yielded clear benefits with few apparent risks. Few would question, for example, the value of our now abundant supply of human insulin produced by genetically engineered bacteria. However, many emerging applications of genetic engineering are much more controversial, often because their potential benefits are pitted against significant risks, real or perceived. This is certainly the case for gene therapy, a clinical application of genetic engineering that may one day provide a cure for many diseases but is still largely an experimental approach to treatment.
Mechanisms and Risks of Gene Therapy
Human diseases that result from genetic mutations are often difficult to treat with drugs or other traditional forms of therapy because the signs and symptoms of disease result from abnormalities in a patient’s genome. For example, a patient may have a genetic mutation that prevents the expression of a specific protein required for the normal function of a particular cell type. This is the case in patients with Severe Combined Immunodeficiency (SCID), a genetic disease that impairs the function of certain white blood cells essential to the immune system.
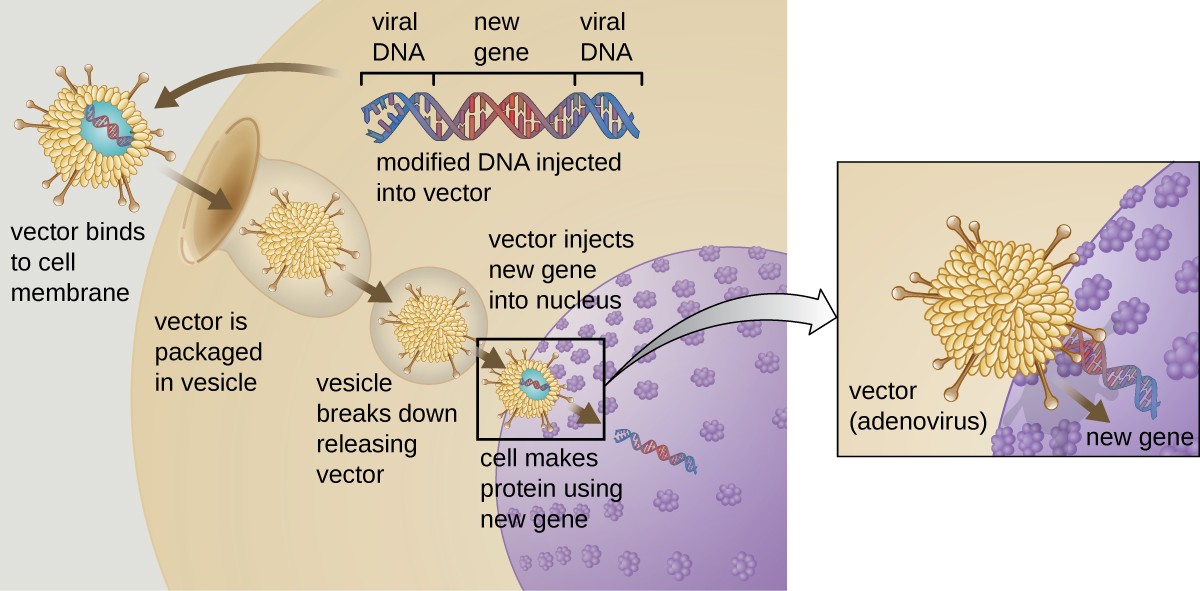
Gene therapy attempts to correct genetic abnormalities by introducing a nonmutated, functional gene into the patient’s genome. The nonmutated gene encodes a functional protein that the patient would otherwise be unable to produce. Viral vectors such as adenovirus are sometimes used to introduce the functional gene; part of the viral genome is
removed and replaced with the desired gene (Figure 12.29). More advanced forms of gene therapy attempt to correct the mutation at the original site in the genome, such as is the case with treatment of SCID.
So far, gene therapies have proven relatively ineffective, with the possible exceptions of treatments for cystic fibrosis and adenosine deaminase deficiency, a type of SCID. Other trials have shown the clear hazards of attempting genetic manipulation in complex multicellular organisms like humans. In some patients, the use of an adenovirus vector can trigger an unanticipated inflammatory response from the immune system, which may lead to organ failure. Moreover, because viruses can often target multiple cell types, the virus vector may infect cells not targeted for the therapy, damaging these other cells and possibly leading to illnesses such as cancer. Another potential risk is that the modified virus could revert to being infectious and cause disease in the patient. Lastly, there is a risk that the inserted gene could unintentionally inactivate another important gene in the patient’s genome, disrupting normal cell cycling and possibly leading to tumor formation and cancer. Because gene therapy involves so many risks, candidates for gene therapy need to be fully informed of these risks before providing informed consent to undergo the therapy.
Case in PointGene Therapy Gone WrongThe risks of gene therapy were realized in the 1999 case of Jesse Gelsinger, an 18-year-old patient who received gene therapy as part of a clinical trial at the University of Pennsylvania. Jesse received gene therapy for a condition called ornithine transcarbamylase (OTC) deficiency, which leads to ammonia accumulation in the blood due to deficient ammonia processing. Four days after the treatment, Jesse died after a massive immune response to the adenovirus vector.[10]Until that point, researchers had not really considered an immune response to the vector to be a legitimate risk, but on investigation, it appears that the researchers had some evidence suggesting that this was a possible outcome. Prior to Jesse’s treatment, several other human patients had suffered side effects of the treatment, and three monkeys used in a trial had died as a result of inflammation and clotting disorders. Despite this information, it appears that neither Jesse nor his family were made aware of these outcomes when they consented to the therapy. Jesse’s death was the first patient death due to a gene therapy treatment and resulted in the immediate halting of the clinical trial in which he was involved, the subsequent halting of all other
gene therapy trials at the University of Pennsylvania, and the investigation of all other gene therapy trials in the United States. As a result, the regulation and oversight of gene therapy overall was reexamined, resulting in new regulatory protocols that are still in place today.

- Explain how gene therapy works in theory.
- Identify some risks of gene therapy.
Oversight of Gene Therapy
Presently, there is significant oversight of gene therapy clinical trials. At the federal level, three agencies regulate gene therapy in parallel: the Food and Drug Administration (FDA), the Office of Human Research Protection (OHRP), and the Recombinant DNA Advisory Committee (RAC) at the National Institutes of Health (NIH). Along with several local agencies, these federal agencies interact with the institutional review board to ensure that protocols are in place to protect patient safety during clinical trials. Compliance with these protocols is enforced mostly on the local level in cooperation with the federal agencies. Gene therapies are currently under the most extensive federal and local review compared to other types of therapies, which are more typically only under the review of the FDA. Some researchers believe that these extensive regulations actually inhibit progress in gene therapy research. In 2013, the Institute of Medicine (now the National Academy of Medicine) called upon the NIH to relax its review of gene therapy trials in most cases.[11] However, ensuring patient safety continues to be of utmost concern.
Ethical Concerns
Beyond the health risks of gene therapy, the ability to genetically modify humans poses a number of ethical issues related to the limits of such “therapy.” While current research is focused on gene therapy for genetic diseases, scientists might one day apply these methods to manipulate other genetic traits not perceived as desirable. This raises questions such as:
- Which genetic traits are worthy of being “corrected”?
- Should gene therapy be used for cosmetic reasons or to enhance human abilities?
- Should genetic manipulation be used to impart desirable traits to the unborn?
- Is everyone entitled to gene therapy, or could the cost of gene therapy create new forms of social inequality?
- Who should be responsible for regulating and policing inappropriate use of gene therapies?
The ability to alter reproductive cells using gene therapy could also generate new ethical dilemmas. To date, the various types of gene therapies have been targeted to somatic cells, the non-reproductive cells within the body. Because somatic cell traits are not inherited, any genetic changes accomplished by somatic-cell gene therapy would not be passed on to offspring. However, should scientists successfully introduce new genes to germ cells (eggs or sperm), the resulting traits could be passed on to offspring. This approach, called germ-line gene therapy, could potentially be used to combat heritable diseases, but it could also lead to unintended consequences for future generations. Moreover, there is the question of informed consent, because those impacted by germ-line gene therapy
- Barbara Sibbald. “Death but One Unintended Consequence of Gene-Therapy Trial.” Canadian Medical Association Journal 164 no. 11 (2001): 1612–1612.
- Kerry Grens. “Report: Ease Gene Therapy Reviews.” The Scientist, December 9, 2013. http://www.the-scientist.com/?articles.view/ articleNo/38577/title/Report–Ease-Gene-Therapy-Reviews/. Accessed May 27, 2016.
are unborn and therefore unable to choose whether they receive the therapy. For these reasons, the U.S. government does not currently fund research projects investigating germ-line gene therapies in humans.
Eye on Ethics
Risky Gene Therapies
While there are currently no gene therapies on the market in the United States, many are in the pipeline and it is likely that some will eventually be approved. With recent advances in gene therapies targeting p53, a gene whose somatic cell mutations have been implicated in over 50% of human cancers,[12] cancer treatments through gene therapies could become much more widespread once they reach the commercial market.
Bringing any new therapy to market poses ethical questions that pit the expected benefits against the risks. How quickly should new therapies be brought to the market? How can we ensure that new therapies have been sufficiently tested for safety and effectiveness before they are marketed to the public? The process by which new therapies are developed and approved complicates such questions, as those involved in the approval process are often under significant pressure to get a new therapy approved even in the face of significant risks.
To receive FDA approval for a new therapy, researchers must collect significant laboratory data from animal trials and submit an Investigational New Drug (IND) application to the FDA’s Center for Drug Evaluation and Research (CDER). Following a 30-day waiting period during which the FDA reviews the IND, clinical trials involving human subjects may begin. If the FDA perceives a problem prior to or during the clinical trial, the FDA can order a “clinical hold” until any problems are addressed. During clinical trials, researchers collect and analyze data on the therapy’s effectiveness and safety, including any side effects observed. Once the therapy meets FDA standards for effectiveness and safety, the developers can submit a New Drug Application (NDA) that details how the therapy will be manufactured, packaged, monitored, and administered.
Because new gene therapies are frequently the result of many years (even decades) of laboratory and clinical research, they require a significant financial investment. By the time a therapy has reached the clinical trials stage, the financial stakes are high for pharmaceutical companies and their shareholders. This creates potential conflicts of interest that can sometimes affect the objective judgment of researchers, their funders, and even trial participants. The Jesse Gelsinger case (see Case in Point: Gene Therapy Gone Wrong) is a classic example. Faced with a life-threatening disease and no reasonable treatments available, it is easy to see why a patient might be eager to participate in a clinical trial no matter the risks. It is also easy to see how a researcher might view the short-term risks for a small group of study participants as a small price to pay for the potential benefits of a game-changing new treatment.
Gelsinger’s death led to increased scrutiny of gene therapy, and subsequent negative outcomes of gene therapy have resulted in the temporary halting of clinical trials pending further investigation. For example, when children in France treated with gene therapy for SCID began to develop leukemia several years after treatment, the FDA temporarily stopped clinical trials of similar types of gene therapy occurring in the United States.[13] Cases like these highlight the need for researchers and health professionals not only to value human well- being and patients’ rights over profitability, but also to maintain scientific objectivity when evaluating the risks and benefits of new therapies.
- Zhen Wang and Yi Sun. “Targeting p53 for Novel Anticancer Therapy.” Translational Oncology 3, no. 1 (2010): 1–12.
- Erika Check. “Gene Therapy: A Tragic Setback.” Nature 420 no. 6912 (2002): 116–118.

- Why is gene therapy research so tightly regulated?
- What is the main ethical concern associated with germ-line gene therapy?
Summary
Microbes and the Tools of Genetic Engineering
- Biotechology is the science of utilizing living systems to benefit humankind. In recent years, the ability to directly alter an organism’s genome through genetic engineering has been made possible due to advances in recombinant DNA technology, which allows researchers to create recombinant DNA molecules with new combinations of genetic material.
- Molecular cloning involves methods used to construct recombinant DNA and facilitate their replication in host organisms. These methods include the use of restriction enzymes (to cut both foreign DNA and plasmid vectors), ligation (to paste fragments of DNA together), and the introduction of recombinant DNA into a host organism (often bacteria).
- Blue-white screening allows selection of bacterial transformants that contain recombinant plasmids using the phenotype of a reporter gene that is disabled by insertion of the DNA fragment.
- Genomic libraries can be made by cloning genomic fragments from one organism into plasmid vectors or into bacteriophage.
- cDNA libraries can be generated to represent the mRNA molecules expressed in a cell at a given point.
- Transfection of eukaryotic hosts can be achieved through various methods using electroporation, gene guns, microinjection, shuttle vectors, and viral vectors.
Visualizing and Characterizing DNA, RNA, and Protein
- Finding a gene of interest within a sample requires the use of a single-stranded DNA probe labeled with a molecular beacon (typically radioactivity or fluorescence) that can hybridize with a complementary single- stranded nucleic acid in the sample.
- Agarose gel electrophoresis allows for the separation of DNA molecules based on size.
- Restriction fragment length polymorphism (RFLP) analysis allows for the visualization by agarose gel electrophoresis of distinct variants of a DNA sequence caused by differences in restriction sites.
- Southern blot analysis allows researchers to find a particular DNA sequence within a sample whereas
northern blot analysis allows researchers to detect a particular mRNA sequence expressed in a sample.
- Microarray technology is a nucleic acid hybridization technique that allows for the examination of many thousands of genes at once to find differences in genes or gene expression patterns between two samples of genomic DNA or cDNA,
- Polyacrylamide gel electrophoresis (PAGE) allows for the separation of proteins by size, especially if native protein charges are masked through pretreatment with SDS.
- Polymerase chain reaction allows for the rapid amplification of a specific DNA sequence. Variations of PCR can be used to detect mRNA expression (reverse transcriptase PCR) or to quantify a particular sequence in the original sample (real-time PCR).
- Although the development of Sanger DNA sequencing was revolutionary, advances in next generation sequencing allow for the rapid and inexpensive sequencing of the genomes of many organisms, accelerating the volume of new sequence data.
Whole Genome Methods and Pharmaceutical Applications of Genetic Engineering
- The science of genomics allows researchers to study organisms on a holistic level and has many applications of medical relevance.
- Transcriptomics and proteomics allow researchers to compare gene expression patterns between different cells and shows great promise in better understanding global responses to various conditions.
- The various –omics technologies complement each other and together provide a more complete picture of an organism’s or microbial community’s (metagenomics) state.
- The analysis required for large data sets produced through genomics, transcriptomics, and proteomics has led to the emergence of bioinformatics.
- Reporter genes encoding easily observable characteristics are commonly used to track gene expression patterns of genes of unknown function.
- The use of recombinant DNA technology has revolutionized the pharmaceutical industry, allowing for the rapid production of high-quality recombinant DNA pharmaceuticals used to treat a wide variety of human conditions.
- RNA interference technology has great promise as a method of treating viral infections by silencing the expression of specific genes
Gene Therapy
- While gene therapy shows great promise for the treatment of genetic diseases, there are also significant risks involved.
- There is considerable federal and local regulation of the development of gene therapies by pharmaceutical companies for use in humans.
- Before gene therapy use can increase dramatically, there are many ethical issues that need to be addressed by the medical and research communities, politicians, and society at large.
Review Questions
Multiple Choice
- Which of the following is required for repairing the phosphodiester backbone of DNA during molecular cloning?
- cDNA
- reverse transcriptase
- restriction enzymes
- DNA ligase
- All of the following are processes used to introduce DNA molecules into bacterial cells except:
- transformation
- transduction
- transcription
- conjugation
- The enzyme that uses RNA as a template to produce a DNA copy is called:
- a restriction enzyme
- DNA ligase
- reverse transcriptase
- DNA polymerase
- In blue-white screening, what do blue colonies represent?
- cells that have not taken up the plasmid vector
- cells with recombinant plasmids containing a new insert
- cells containing empty plasmid vectors
- cells with a non-functional lacZ gene
- The Ti plasmid is used for introducing genes into:
- animal cells
- plant cells
- bacteriophages
- E. coli cells
- Which technique is used to separate protein fragments based on size?
- polyacrylamide gel electrophoresis
- Southern blot
- agarose gel electrophoresis
- polymerase chain reaction
- Which technique uses restriction enzyme digestion followed by agarose gel electrophoresis to generate a banding pattern for comparison to another sample processed in the same way?
- qPCR
- RT-PCR
- RFLP
- 454 sequencing
- All of the following techniques involve hybridization between single-stranded nucleic acid molecules except:
- Southern blot analysis
- RFLP analysis
- northern blot analysis
- microarray analysis
- The science of studying the entire collection of mRNA molecules produced by cells, allowing scientists to monitor differences in gene expression patterns between cells, is called:
- genomics
- transcriptomics
- proteomics
- pharmacogenomics
- The science of studying genomic fragments from microbial communities, allowing researchers to study genes from a collection of multiple species, is called:
- pharmacogenomics
- transcriptomics
- metagenomics
- proteomics
- The insulin produced by recombinant DNA technology is
- a combination of E. coli and human insulin.
- identical to human insulin produced in the pancreas.
- cheaper but less effective than pig insulin for treating diabetes.
- engineered to be more effective than human insulin.
- At what point can the FDA halt the development or use of gene therapy?
- on submission of an IND application
- during clinical trials
- after manufacturing and marketing of the approved therapy
- all of the answers are correct
True/False
- Recombination is a process not usually observed in nature.
- It is generally easier to introduce recombinant DNA into prokaryotic cells than into eukaryotic cells.
- In agarose gel electrophoresis, DNA will be attracted to the negative electrode.
- RNA interference does not influence the sequence of genomic DNA.
Fill in the Blank
- The process of introducing DNA molecules into eukaryotic cells is called .
- The blot technique is used to find an RNA fragment within a sample that is complementary to a DNA probe.
- The PCR step during which the double-stranded template molecule becomes single-stranded is called
.
- The sequencing method involving the incorporation of ddNTPs is called .
- The application of genomics to evaluate the effectiveness and safety of drugs on the basis of information from an individual’s genomic sequence is called .
- A gene whose expression can be easily visualized and monitored is called a .
- is a common viral vector used in gene therapy for introducing a new gene into a specifically targeted cell type.
Short Answer
- Name three elements incorporated into a plasmid vector for efficient cloning.
- When would a scientist want to generate a cDNA library instead of a genomic library?
- What is one advantage of generating a genomic library using phages instead of plasmids?
- Why is it important that a DNA probe be labeled with a molecular beacon?
- When separating proteins strictly by size, why is exposure to SDS first required?
- Why must the DNA polymerase used during PCR be heat-stable?
- If all cellular proteins are encoded by the cell’s genes, what information does proteomics provide that genomics cannot?
- Briefly describe the risks associated with somatic cell gene therapy.
Critical Thinking
- Is biotechnology always associated with genetic engineering? Explain your answer.
- Which is more efficient: blunt-end cloning or sticky-end cloning? Why?
- Suppose you are working in a molecular biology laboratory and are having difficulty performing the PCR successfully. You decide to double-check the PCR protocol programmed into the thermal cycler and discover that the annealing temperature was programmed to be 65 °C instead of 50 °C, as you had intended. What effects would this mistake have on the PCR reaction? Refer to Figure 12.20.
- What is the advantage of microarray analysis over northern blot analysis in monitoring changes in gene expression?
- What is the difference between reverse transcriptase PCR (RT-PCR) and real-time quantitative PCR (qPCR)?
- What are some advantages of cloning human genes into bacteria to treat human diseases caused by specific protein deficiencies?
- Compare the ethical issues involved in the use of somatic cell gene therapy and germ-line gene therapy.

