Chapter 7 Introduction – Microbial Biochemistry
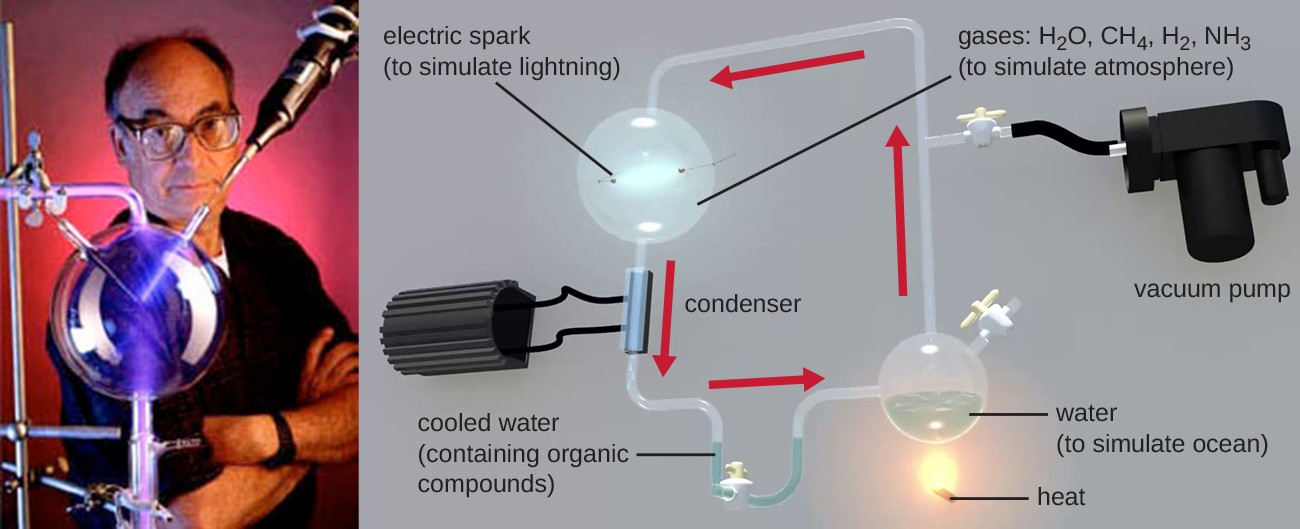
The earth is estimated to be 4.6 billion years old, but for the first 2 billion years, the atmosphere lacked oxygen, without which the earth could not support life as we know it. One hypothesis about how life emerged on earth involves the concept of a “primordial soup.” This idea proposes that life began in a body of water when metals and gases from the atmosphere combined with a source of energy, such as lightning or ultraviolet light, to form the carbon compounds that are the chemical building blocks of life. In 1952, Stanley Miller (1930–2007), a graduate student at the University of Chicago, and his professor Harold Urey (1893–1981), set out to confirm this hypothesis in a now- famous experiment. Miller and Urey combined what they believed to be the major components of the earth’s early atmosphere—water (H2O), methane (CH4), hydrogen (H2), and ammonia (NH3)—and sealed them in a sterile flask. Next, they heated the flask to produce water vapor and passed electric sparks through the mixture to mimic lightning in the atmosphere (Figure 7.1). When they analyzed the contents of the flask a week later, they found amino acids, the structural units of proteins—molecules essential to the function of all organisms.

