5.2 – Parasitic Helminths
Learning Objectives
- Explain why we include the study of parasitic worms within the discipline of microbiology
- Compare the basic morphology of the major groups of parasitic helminthes
- Describe the characteristics of parasitic nematodes, and give an example of infective eggs and infective larvae
- Describe the characteristics of parasitic trematodes and cestodes, and give examples of each
- Identify examples of the primary causes of infections due to nematodes, trematodes, and cestodes
- Classify parasitic worms according to major groups
Parasitic helminths are animals that are often included within the study of microbiology because many species of these worms are identified by their microscopic eggs and larvae. There are two major groups of parasitic helminths: the roundworms (Nematoda) and flatworms (Platyhelminthes). Of the many species that exist in these groups, about half are parasitic and some are important human pathogens. As animals, they are multicellular and have organ systems. However, the parasitic species often have limited digestive tracts, nervous systems, and locomotor abilities. Parasitic forms may have complex reproductive cycles with several different life stages and more than one type of host. Some are monoecious, having both male and female reproductive organs in a single individual, while others are dioecious, each having either male or female reproductive organs.
Nematoda (Roundworms)
Phylum Nematoda (the roundworms) is a diverse group containing more than 15,000 species, of which several are important human parasites (Figure 5.19). These unsegmented worms have a full digestive system even when parasitic. Some are common intestinal parasites, and their eggs can sometimes be identified in feces or around the anus of infected individuals. Ascaris lumbricoides is the largest nematode intestinal parasite found in humans; females may reach lengths greater than 1 meter. A. lumbricoides is also very widespread, even in developed nations, although it is now a relatively uncommon problem in the United States. It may cause symptoms ranging from relatively mild (such as a cough and mild abdominal pain) to severe (such as intestinal blockage and impaired growth)

Of all nematode infections in the United States, pinworm (caused by Enterobius vermicularis) is the most common. Pinworm causes sleeplessness and itching around the anus, where the female worms lay their eggs during the night. Toxocara canis and T. cati are nematodes found in dogs and cats, respectively, that can be transmitted to humans, causing toxocariasis. Antibodies to these parasites have been found in approximately 13.9% of the U.S. population, suggesting that exposure is common.7 Infection can cause larval migrans, which can result in vision loss and eye inflammation, or fever, fatigue, coughing, and abdominal pain, depending on whether the organism infects the eye or the viscera. Another common nematode infection is hookworm, which is caused by Necator americanus (the New World or North American hookworm) and Ancylostoma duodenale (the Old World hookworm). Symptoms of hookworm infection can include abdominal pain, diarrhea, loss of appetite, weight loss, fatigue, and anemia.
Trichinellosis, also called trichinosis, caused by Trichinella spiralis, is contracted by consuming undercooked meat, which releases the larvae and allows them to encyst in muscles. Infection can cause fever, muscle pains, and digestive system problems; severe infections can lead to lack of coordination, breathing and heart problems, and even death. Finally, heartworm in dogs and other animals is caused by the nematode Dirofilaria immitis, which is transmitted by mosquitoes. Symptoms include fatigue and cough; when left untreated, death may result.
Platyhelminths (Flatworms)
Phylum Platyhelminthes (the platyhelminths) are flatworms. This group includes the flukes, tapeworms, and the turbellarians, which include planarians. The flukes and tapeworms are medically important parasites (Figure 5.20).
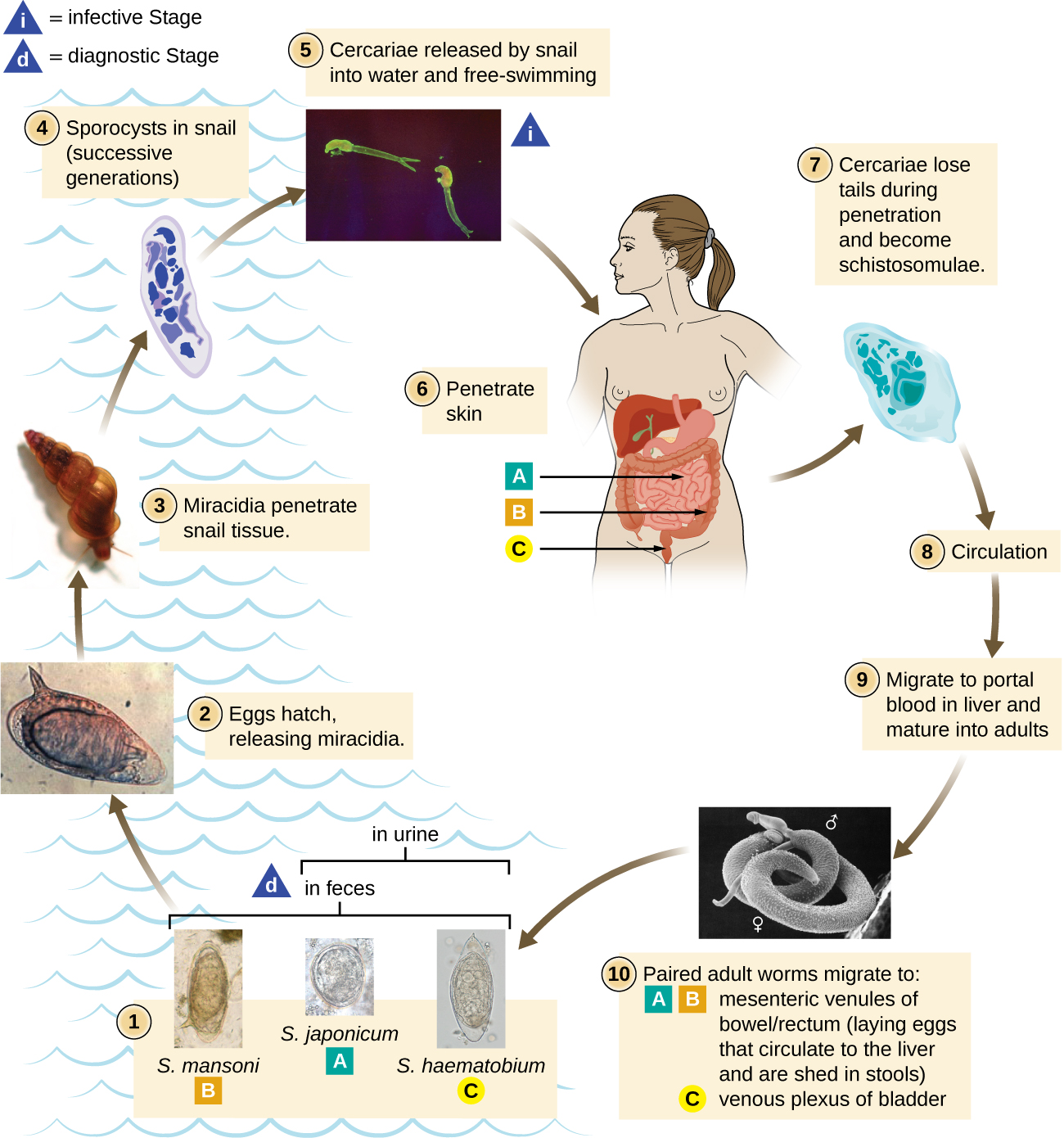
The flukes (trematodes) are nonsegmented flatworms that have an oral sucker (Figure 5.21) (and sometimes a second ventral sucker) and attach to the inner walls of intestines, lungs, large blood vessels, or the liver. Trematodes have complex life cycles, often with multiple hosts. Several important examples are the liver flukes (Clonorchis and Opisthorchis), the intestinal fluke (Fasciolopsis buski), and the oriental lung fluke (Paragonimus westermani). Schistosomiasis is a serious parasitic disease, considered second in the scale of its impact on human populations only to malaria. The parasites Schistosoma mansoni, S. haematobium, and S. japonicum, which are found in freshwater snails, are responsible for schistosomiasis (Figure 5.22). Immature forms burrow through the skin into the blood. They migrate to the lungs, then to the liver and, later, other organs. Symptoms include anemia, malnutrition, fever, abdominal pain, fluid buildup, and sometimes death.
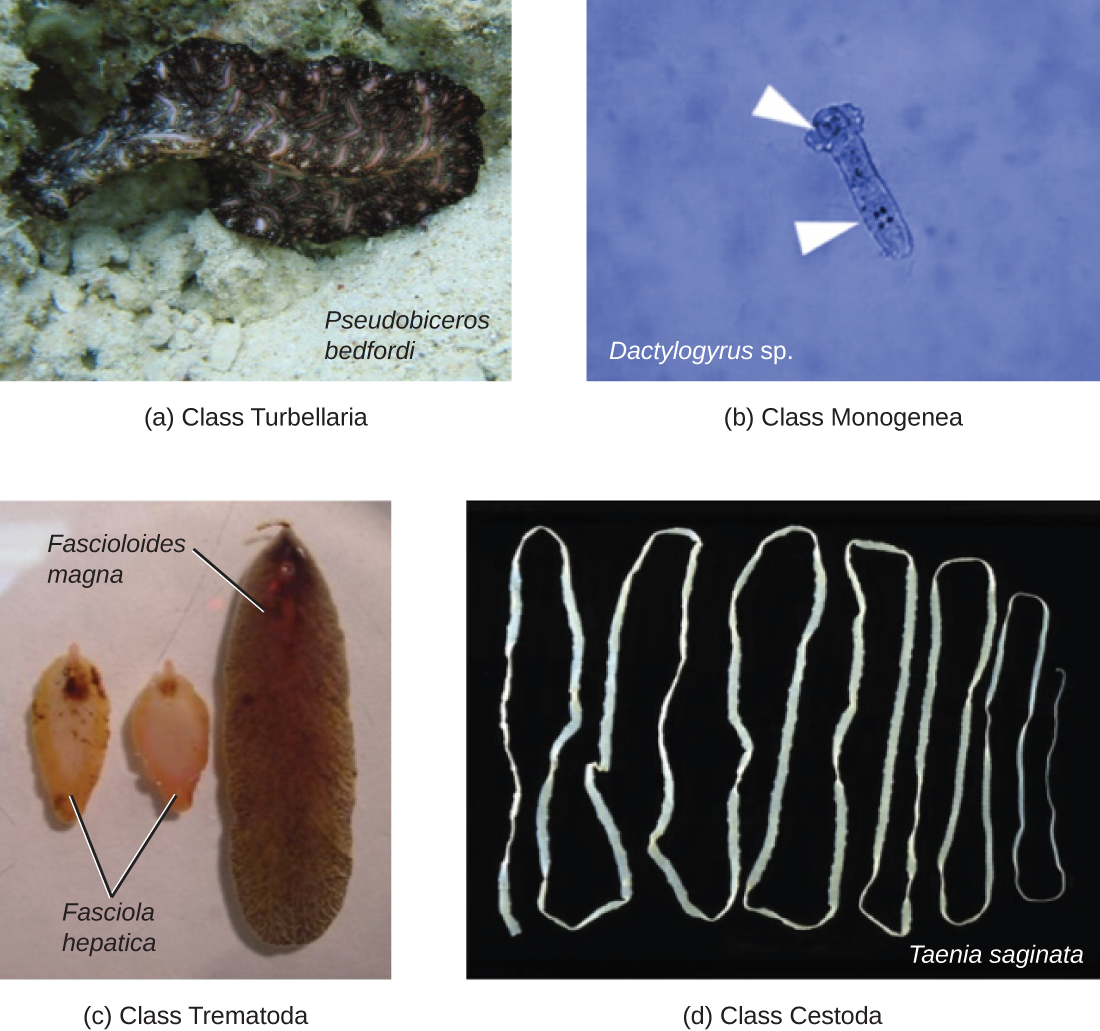
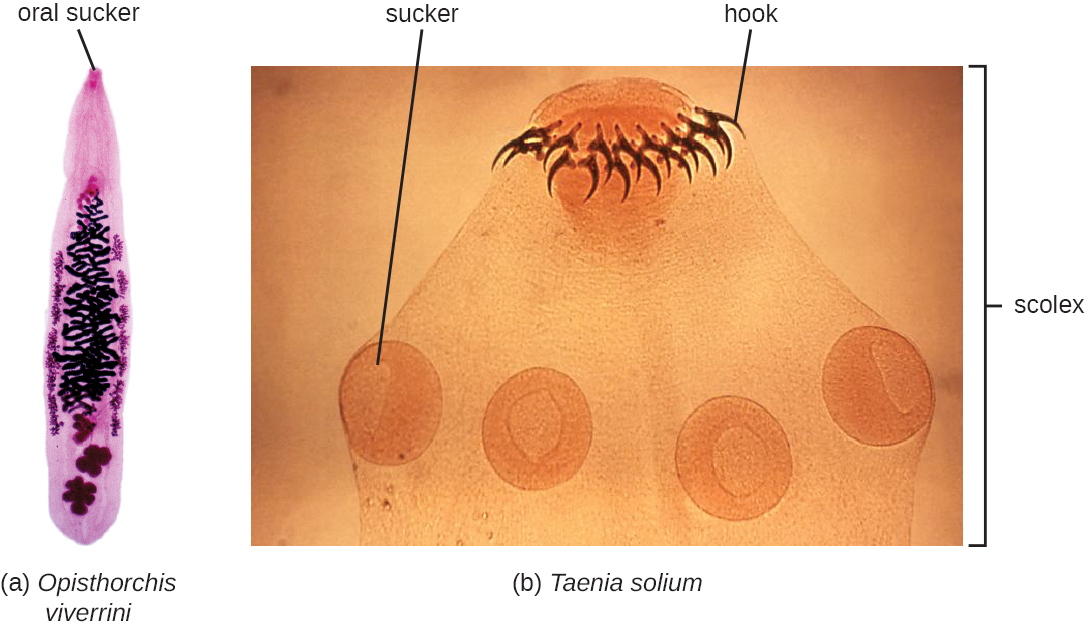
The other medically important group of platyhelminths are commonly known as tapeworms (cestodes) and are segmented flatworms that may have suckers or hooks at the scolex (head region) (Figure 5.21). Tapeworms use these suckers or hooks to attach to the wall of the small intestine. The body of the worm is made up of segments called proglottids that contain reproductive structures; these detach when the gametes are fertilized, releasing gravid proglottids with eggs. Tapeworms often have an intermediate host that consumes the eggs, which then hatch into a larval form called an oncosphere. The oncosphere migrates to a particular tissue or organ in the intermediate host, where it forms cysticerci. After being eaten by the definitive host, the cysticerci develop into adult tapeworms in the host’s digestive system (Figure 5.23). Taenia saginata (the beef tapeworm) and T. solium (the pork tapeworm) enter humans through ingestion of undercooked, contaminated meat. The adult worms develop and reside in the intestine, but the larval stage may migrate and be found in other body locations such as skeletal and smooth muscle. The beef tapeworm is relatively benign, although it can cause digestive problems and, occasionally, allergic reactions. The pork tapeworm can cause more serious problems when the larvae leave the intestine and colonize other tissues, including those of the central nervous system. Diphylobothrium latum is the largest human tapeworm and can be ingested in undercooked fish. It can grow to a length of 15 meters. Echinococcus granulosus, the dog tapeworm, can parasitize humans and uses dogs as an important host.
Check Your Understanding
- What group of medically important flatworms is segmented and what group is unsegmented?
Micro Connections
Food for Worms?
For residents of temperate, developed countries, it may be difficult to imagine just how common helminth infections are in the human population. In fact, they are quite common and even occur frequently in the United States. Worldwide, approximately 807–1,221 million people are infected with Ascaris lumbricoides (perhaps one-sixth of the human population) and far more are infected if all nematode species are considered.8 Rates of infection are relatively high even in industrialized nations. Approximately 604–795 million people are infected with whipworm (Trichuris) worldwide (Trichuris can also infect dogs), and 576–740 million people are infected with hookworm (Necator americanus and Ancylostoma duodenale).9 Toxocara, a nematode parasite of dogs and cats, is also able to infect humans. It is widespread in the United States, with about 10,000 symptomatic cases annually. However, one study found 14% of the population (more than 40 million Americans) was seropositive, meaning they had been exposed to the parasite at one time. More than 200 million people have schistosomiasis worldwide. Most of the World Health Organization (WHO) neglected tropical diseases are helminths. In some cases, helminths may cause subclinical illnesses, meaning the symptoms are so mild that that they go unnoticed. In other cases, the effects may be more severe or chronic, leading to fluid accumulation and organ damage. With so many people affected, these parasites constitute a major global public health concern.
Micro Connections
Eradicating the Guinea Worm
Dracunculiasis, or Guinea worm disease, is caused by a nematode called Dracunculus medinensis. When people consume contaminated water, water fleas (small crustaceans) containing the nematode larvae may be ingested. These larvae migrate out of the intestine, mate, and move through the body until females eventually emerge (generally through the feet). While Guinea worm disease is rarely fatal, it is extremely painful and can be accompanied by secondary infections and edema (Figure 5.23).
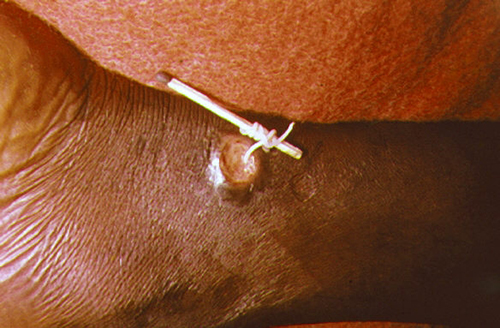
An eradication campaign led by WHO, the CDC, the United Nations Children’s Fund (UNICEF), and the Carter Center (founded by former U.S. president Jimmy Carter) has been extremely successful in reducing cases of dracunculiasis. This has been possible because diagnosis is straightforward, there is an inexpensive method of control, there is no animal reservoir, the water fleas are not airborne (they are restricted to still water), the disease is geographically limited, and there has been a commitment from the governments involved. Additionally, no vaccines or medication are required for treatment and prevention. In 1986, 3.5 million people were estimated to be affected. After the eradication campaign, which included helping people in affected areas learn to filter water with cloth, only four countries continue to report the disease (Chad, Mali, South Sudan, and Ethiopia) with a total of 126 cases reported to WHO in 2014.10
Footnotes
Won K, Kruszon-Moran D, Schantz P, Jones J. “National seroprevalence and risk factors for zoonotic Toxocara spp. infection.” In: Abstracts of the 56th American Society of Tropical Medicine and Hygiene; Philadelphia, Pennsylvania; 2007 Nov 4-8.
Fenwick, A. “The global burden of neglected tropical diseases.” Public health 126 no.3 (Mar 2012): 233–6.
de Silva, N., et. al. (2003). “Soil-transmitted helminth infections: updating the global picture”. Trends in Parasitology 19 (December 2003): 547–51.
World Health Organization. “South Sudan Reports Zero Cases of Guinea-Worm Disease for Seventh Consecutive Month.” 2016. http://www.who.int/dracunculiasis/no_new_case_for_seventh_consecutive_months/en/. Accessed May 2, 2016.

