Plants
19.1 – Evolution of Seed Plants
Learning Objectives
By the end of this section, you will be able to do the following:
- Describe the two major innovations that allowed seed plants to reproduce in the absence of water
- Explain when seed plants first appeared and when gymnosperms became the dominant plant group
- Discuss the purpose of pollen grains and seeds
- Describe the significance of angiosperms bearing both flowers and fruit
The first plants to colonize land were most likely related to the ancestors of modern day mosses (bryophytes), which are thought to have appeared about 500 million years ago. They were followed by liverworts (also bryophytes) and primitive vascular plants—the pterophytes—from which modern ferns are descended. The life cycle of bryophytes and pterophytes is characterized by the alternation of generations, which is also exhibited in the gymnosperms and angiosperms. However, what sets bryophytes and pterophytes apart from gymnosperms and angiosperms is their reproductive requirement for water. The completion of the bryophyte and pterophyte life cycle requires water because the male gametophyte releases flagellated sperm, which must swim to reach and fertilize the female gamete or egg. After fertilization, the zygote undergoes cellular division and grows into a diploid sporophyte, which in turn will form sporangia or “spore vessels.” In the sporangia, mother cells undergo meiosis and produce the haploid spores. Release of spores in a suitable environment will lead to germination and a new generation of gametophytes.
In seed plants, the evolutionary trend led to a dominant sporophyte generation accompanied by a corresponding reduction in the size of the gametophyte from a conspicuous structure to a microscopic cluster of cells enclosed in the tissues of the sporophyte. Whereas lower vascular plants, such as club mosses and ferns, are mostly homosporous (producing only one type of spore), all seed plants, or spermatophytes, are heterosporous, producing two types of spores: megaspores (female) and microspores (male). Megaspores develop into female gametophytes that produce eggs, and microspores mature into male gametophytes that generate sperm. Because the gametophytes mature within the spores, they are not free-living, as are the gametophytes of other seedless vascular plants.
Ancestral heterosporous seedless plants, represented by modern-day plants such as the spike moss Selaginella, are seen as the evolutionary forerunners of seed plants. In the life cycle of Selaginella, both male and female sporangia develop within the same stem-like strobilus. In each male sporangium, multiple microspores are produced by meiosis. Each microspore produces a small antheridium contained within a spore case. As it develops it is released from the strobilus, and a number of flagellated sperm are produced that then leave the spore case. In the female sporangium, a single megaspore mother cell undergoes meiosis to produce four megaspores. Gametophytes develop within each megaspore, consisting of a mass of tissue that will later nourish the embryo and a few archegonia. The female gametophyte may remain within remnants of the spore wall in the megasporangium until after fertilization has occurred and the embryo begins to develop. This combination of an embryo and nutritional cells is a little different from the organization of a seed, since the nutritive endosperm in a seed is formed from a single cell rather than multiple cells.
Both seeds and pollen distinguish seed plants from seedless vascular plants. These innovative structures allowed seed plants to reduce or eliminate their dependence on water for gamete fertilization and development of the embryo, and to conquer dry land. Pollen grains are male gametophytes, which contain the sperm (gametes) of the plant. The small haploid (1n) cells are encased in a protective coat that prevents desiccation (drying out) and mechanical damage. Pollen grains can travel far from their original sporophyte, spreading the plant’s genes. Seeds offer the embryo protection, nourishment, and a mechanism to maintain dormancy for tens or even thousands of years, ensuring that germination can occur when growth conditions are optimal. Seeds therefore allow plants to disperse the next generation through both space and time. With such evolutionary advantages, seed plants have become the most successful and familiar group of plants.
Both adaptations expanded the colonization of land begun by the bryophytes and their ancestors. Fossils place the earliest distinct seed plants at about 350 million years ago. The first reliable record of gymnosperms dates their appearance to the Pennsylvanian period, about 319 million years ago ((Figure)). Gymnosperms were preceded by progymnosperms, the first naked seed plants, which arose about 380 million years ago. Progymnosperms were a transitional group of plants that superficially resembled conifers (cone bearers) because they produced wood from the secondary growth of the vascular tissues; however, they still reproduced like ferns, releasing spores into the environment. At least some species were heterosporous. Progymnosperms, like the extinct Archaeopteris (not to be confused with the ancient bird Archaeopteryx), dominated the forests of the late Devonian period. However, by the early (Triassic, c. 240 MYA) and middle (Jurassic, c. 205 MYA) Mesozoic era, the landscape was dominated by the true gymnosperms. Angiosperms surpassed gymnosperms by the middle of the Cretaceous (c. 100 MYA) in the late Mesozoic era, and today are the most abundant and biologically diverse plant group in most terrestrial biomes.
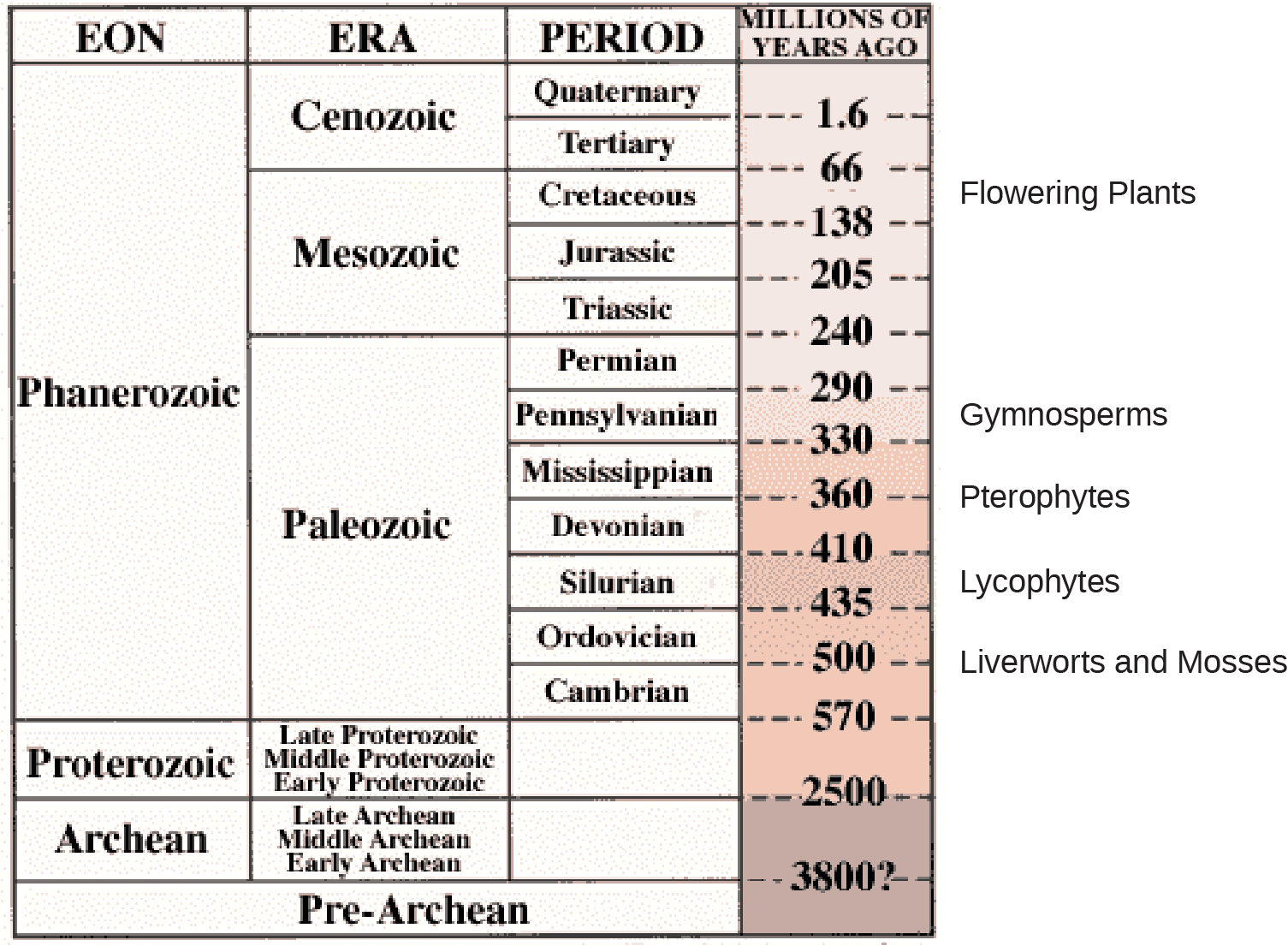
Evolution of Gymnosperms
The fossil plant Elkinsia polymorpha, a “seed fern” from the Devonian period—about 400 million years ago—is considered the earliest seed plant known to date. Seed ferns ((Figure)) produced their seeds along their branches, in structures called cupules that enclosed and protected the ovule—the female gametophyte and associated tissues—which develops into a seed upon fertilization. Seed plants resembling modern tree ferns became more numerous and diverse in the coal swamps of the Carboniferous period.

Fossil records indicate the first gymnosperms (progymnosperms) most likely originated in the Paleozoic era, during the middle Devonian period: about 390 million years ago. The previous Mississippian and Pennsylvanian periods, were wet and dominated by giant fern trees. But the following Permian period was dry, which gave a reproductive edge to seed plants, which are better adapted to survive dry spells. The Ginkgoales, a group of gymnosperms with only one surviving species—the Ginkgo biloba—were the first gymnosperms to appear during the lower Jurassic. Gymnosperms expanded in the Mesozoic era (about 240 million years ago), supplanting ferns in the landscape, and reaching their greatest diversity during this time. The Jurassic period was as much the age of the cycads (palm-tree-like gymnosperms) as the age of the dinosaurs. Ginkgoales and the more familiar conifers also dotted the landscape. Although angiosperms (flowering plants) are the major form of plant life in most biomes, gymnosperms still dominate some ecosystems, such as the taiga (boreal forests) and the alpine forests at higher mountain elevations ((Figure)) because of their adaptation to cold and dry growth conditions.

Seeds and Pollen as an Evolutionary Adaptation to Dry Land
Bryophyte and fern spores are haploid cells dependent on moisture for rapid development of multicellular gametophytes. In the seed plants, the female gametophyte consists of just a few cells: the egg and some supportive cells, including the endosperm-producing cell that will support the growth of the embryo. After fertilization of the egg, the diploid zygote produces an embryo that will grow into the sporophyte when the seed germinates. Storage tissue to sustain growth of the embryo and a protective coat give seeds their superior evolutionary advantage. Several layers of hardened tissue prevent desiccation, and free the embryo from the need for a constant supply of water. Furthermore, seeds remain in a state of dormancy—induced by desiccation and the hormone abscisic acid—until conditions for growth become favorable. Whether blown by the wind, floating on water, or carried away by animals, seeds are scattered in an expanding geographic range, thus avoiding competition with the parent plant.
Pollen grains ((Figure)) are male gametophytes containing just a few cells and are distributed by wind, water, or an animal pollinator. The whole structure is protected from desiccation and can reach the female organs without depending on water. After reaching a female gametophyte, the pollen grain grows a tube that will deliver a male nucleus to the egg cell. The sperm of modern gymnosperms and all angiosperms lack flagella, but in cycads, Ginkgo, and other primitive gymnosperms, the sperm are still motile, and use flagella to swim to the female gamete; however, they are delivered to the female gametophyte enclosed in a pollen grain. The pollen grows or is taken into a fertilization chamber, where the motile sperm are released and swim a short distance to an egg.
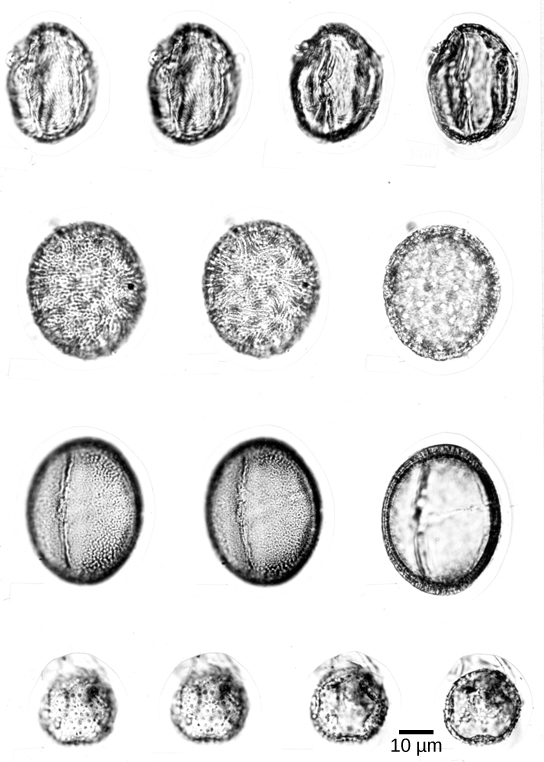
Evolution of Angiosperms
The roughly 200 million years between the appearance of the gymnosperms and the flowering plants gives us some appreciation for the evolutionary experimentation that ultimately produced flowers and fruit. Angiosperms (“seed in a vessel”) produce a flower containing male and/or female reproductive structures. Fossil evidence ((Figure)) indicates that flowering plants first appeared about 125 million years ago in the Lower Cretaceous (late in the Mesozoic era), and were rapidly diversifying by about 100 million years ago in the Middle Cretaceous. Earlier traces of angiosperms are scarce. Fossilized pollen recovered from Jurassic geological material has been attributed to angiosperms. A few early Cretaceous rocks show clear imprints of leaves resembling angiosperm leaves. By the mid-Cretaceous, a staggering number of diverse flowering plants crowd the fossil record. The same geological period is also marked by the appearance of many modern groups of insects, suggesting that pollinating insects played a key role in the evolution of flowering plants.
New data in comparative genomics and paleobotany (the study of ancient plants) have shed some light on the evolution of angiosperms. Although the angiosperms appeared after the gymnosperms, they are probably not derived from gymnosperm ancestors. Instead, the angiosperms form a sister clade (a species and its descendents) that developed in parallel with the gymnosperms. The two innovative structures of flowers and fruit represent an improved reproductive strategy that served to protect the embryo, while increasing genetic variability and range. There is no current consensus on the origin of the angiosperms. Paleobotanists debate whether angiosperms evolved from small woody bushes, or were related to the ancestors of tropical grasses. Both views draw support from cladistics, and the so-called woody magnoliid hypothesis—which proposes that the early ancestors of angiosperms were shrubs like modern magnolia—also offers molecular biological evidence.
The most primitive living angiosperm is considered to be Amborella trichopoda, a small plant native to the rainforest of New Caledonia, an island in the South Pacific. Analysis of the genome of A. trichopoda has shown that it is related to all existing flowering plants and belongs to the oldest confirmed branch of the angiosperm family tree. The nuclear genome shows evidence of an ancient whole-genome duplication. The mitochondrial genome is large and multichromosomal, containing elements from the mitochondrial genomes of several other species, including algae and a moss. A few other angiosperm groups, called basal angiosperms, are viewed as having ancestral traits because they branched off early from the phylogenetic tree. Most modern angiosperms are classified as either monocots or eudicots, based on the structure of their leaves and embryos. Basal angiosperms, such as water lilies, are considered more ancestral in nature because they share morphological traits with both monocots and eudicots.

Flowers and Fruits as an Evolutionary Adaptation
Angiosperms produce their gametes in separate organs, which are usually housed in a flower. Both fertilization and embryo development take place inside an anatomical structure that provides a stable system of sexual reproduction largely sheltered from environmental fluctuations. With about 300,000 species, flowering plants are the most diverse phylum on Earth after insects, which number about 1,200,000 species. Flowers come in a bewildering array of sizes, shapes, colors, smells, and arrangements. Most flowers have a mutualistic pollinator, with the distinctive features of flowers reflecting the nature of the pollination agent. The relationship between pollinator and flower characteristics is one of the great examples of coevolution.
Following fertilization of the egg, the ovule grows into a seed. The surrounding tissues of the ovary thicken, developing into a fruit that will protect the seed and often ensure its dispersal over a wide geographic range. Not all fruits develop completely from an ovary; such “false fruits” or pseudocarps, develop from tissues adjacent to the ovary. Like flowers, fruit can vary tremendously in appearance, size, smell, and taste. Tomatoes, green peppers, corn, and avocados are all examples of fruits. Along with pollen and seeds, fruits also act as agents of dispersal. Some may be carried away by the wind. Many attract animals that will eat the fruit and pass the seeds through their digestive systems, then deposit the seeds in another location. Cockleburs are covered with stiff, hooked spines that can hook into fur (or clothing) and hitch a ride on an animal for long distances. The cockleburs that clung to the velvet trousers of an enterprising Swiss hiker, George de Mestral, inspired his invention of the loop and hook fastener he named Velcro.
Evolution Connection
Building Phylogenetic Trees with Analysis of DNA Sequence AlignmentsAll living organisms display patterns of relationships derived from their evolutionary history. Phylogeny is the science that describes the relative connections between organisms, in terms of ancestral and descendant species. Phylogenetic trees, such as the plant evolutionary history shown in (Figure), are tree-like branching diagrams that depict these relationships. Species are found at the tips of the branches. Each branching point, called a node, is the point at which a single taxonomic group (taxon), such as a species, separates into two or more species.
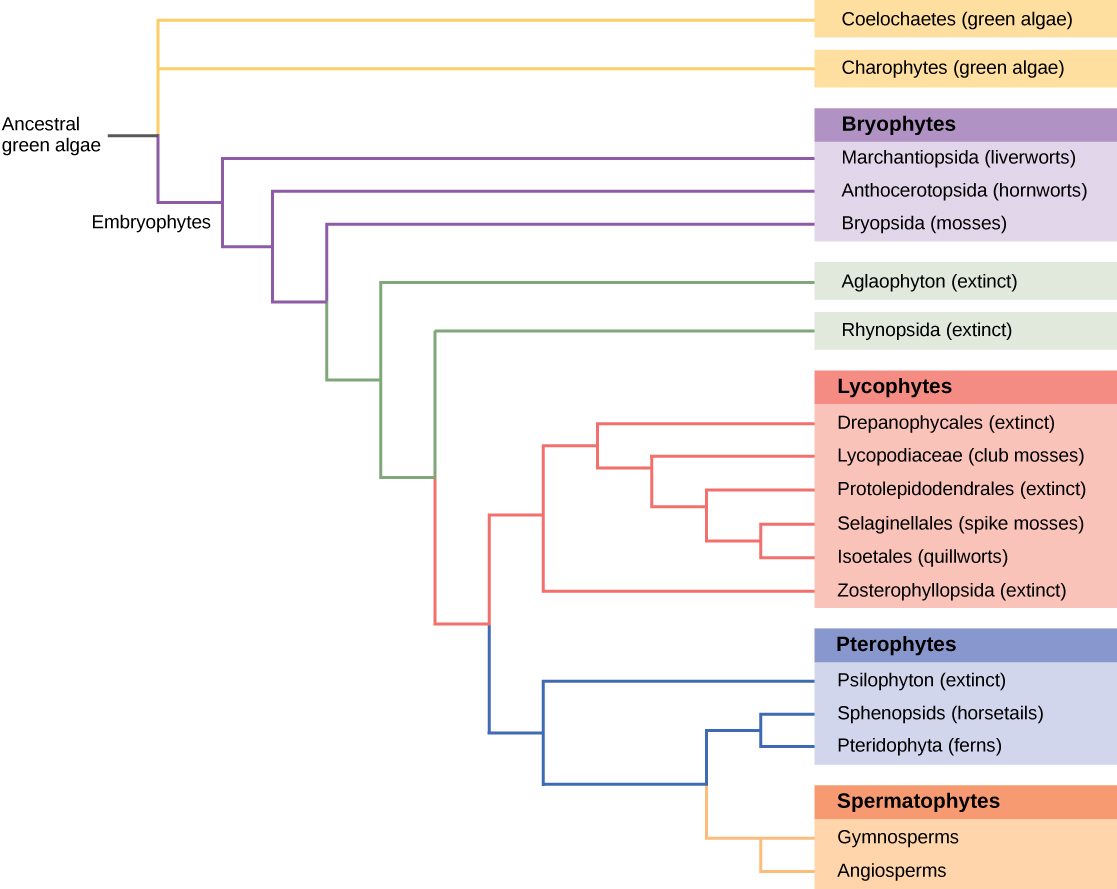
Phylogenetic trees have been built to describe the relationships between species since the first sketch of a tree that appeared in Darwin’s Origin of Species. Traditional methods involve comparison of homologous anatomical structures and embryonic development, assuming that closely related organisms share anatomical features that emerge during embryo development. Some traits that disappear in the adult are present in the embryo; for example, an early human embryo has a postanal tail, as do all members of the Phylum Chordata. The study of fossil records shows the intermediate stages that link an ancestral form to its descendants. However, many of the approaches to classification based on the fossil record alone are imprecise and lend themselves to multiple interpretations. As the tools of molecular biology and computational analysis have been developed and perfected in recent years, a new generation of tree-building methods has taken shape. The key assumption is that genes for essential proteins or RNA structures, such as the ribosomal RNAs, are inherently conserved because mutations (changes in the DNA sequence) could possibly compromise the survival of the organism. DNA from minute samples of living organisms or fossils can be amplified by polymerase chain reaction (PCR) and sequenced, targeting the regions of the genome that are most likely to be conserved between species. The genes encoding the 18S ribosomal RNA from the small subunit and plastid genes are frequently chosen for DNA alignment analysis.
Once the sequences of interest are obtained, they are compared with existing sequences in databases such as GenBank, which is maintained by The National Center for Biotechnology Information. A number of computational tools are available to align and analyze sequences. Sophisticated computer analysis programs determine the percentage of sequence identity or homology. Sequence homology can be used to estimate the evolutionary distance between two DNA sequences and reflect the time elapsed since the genes separated from a common ancestor. Molecular analysis has revolutionized phylogenetic trees. In some cases, prior results from morphological studies have been confirmed: for example, confirming Amborella trichopoda as the most primitive angiosperm known. However, some groups and relationships have been rearranged as a result of DNA analysis.
Section Summary
Seed plants appeared about one million years ago, during the Carboniferous period. Two major innovations were seeds and pollen. Seeds protect the embryo from desiccation and provide it with a store of nutrients to support the early growth of the sporophyte. Seeds are also equipped to delay germination until growth conditions are optimal. Pollen allows seed plants to reproduce in the absence of water. The gametophytes of seed plants shrank, while the sporophytes became prominent structures and the diploid stage became the longest phase of the life cycle.
In the gymnosperms, which appeared during the drier Permian period and became the dominant group during the Triassic, pollen was dispersed by wind, and their naked seeds developed in the sporophylls of a strobilus. Angiosperms bear both flowers and fruit. Flowers expand the possibilities for pollination, especially by insects, who have coevolved with the flowering plants. Fruits offer additional protection to the embryo during its development, and also assist with seed dispersal. Angiosperms appeared during the Mesozoic era and have become the dominant plant life in terrestrial habitats.
Glossary
- flower
- branches specialized for reproduction found in some seed-bearing plants, containing either specialized male or female organs or both male and female organs
- fruit
- thickened tissue derived from ovary wall that protects the embryo after fertilization and facilitates seed dispersal
- ovule
- female gametophyte
- pollen grain
- structure containing the male gametophyte of the plant
- pollen tube
- extension from the pollen grain that delivers sperm to the egg cell
- progymnosperm
- transitional group of plants that resembled conifers because they produced wood, yet still reproduced like ferns
- seed
- structure containing the embryo, storage tissue, and protective coat
- spermatophyte
- seed plant; from the Greek sperm (seed) and phyte (plant)

