Chapter 7: Healthy Eating
How Food Choices Affect Stress
Introduction
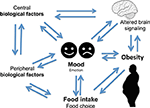
It is hypothesized that individuals engage in a variety of behaviors to regulate their mood (Morris and Reilly, 1987). Important among mood regulating behaviors is food consumption. The interaction between mood, emotional state, and feeding behaviors is complex and it is hypothesized that individuals regulate their emotions and mood by changing both food choices and quantities. It is also apparent that mood can affect the self-rewarding mechanisms of food consumption (Morris and Reilly, 1987). Specific types of food tend to be preferred under certain psychological conditions due to the influence of foods on the activity of brain reward centers (Figure 1) (Rangel, 2013; Jauch-Chara and Oltmanns, 2014; Weltens et al., 2014). Positive feedback loops can result in enhancement of appetite leading to obesity. Interestingly, highly palatable foods activate the same brain regions of reward and pleasure that are active in drug addiction (Volkow et al., 2012), suggesting a neuronal mechanism of food addiction leading to overeating and obesity (Davis et al., 2011, 2014; Dileone et al., 2012; Volkow et al., 2012; Dagher, 2013; Davis, 2013; Ziauddeen and Fletcher, 2013; Pai et al., 2014; Potenza, 2014). Dopamine, which directly activates reward and pleasure centers, affects both mood and food intake (Cantello et al., 1989; Diehl and Gershon, 1992; Fochtmann and Fink, 1992; Black et al., 2002; Cawley et al., 2013), further supporting the link between psychology and eating behaviors.
Mood disorders are often found in association with abnormal feeding behaviors. For example, depression and anxiety are comorbidities of obesity (Novick et al., 2005; Simon et al., 2006; Kloiber et al., 2007). Impairment in central nervous system (CNS) function has been linked to obesity that in turn impacts mental and physical health (Allison et al., 2009; Talen and Mann, 2009; Duarte et al., 2010). Obese individuals are at increased risk of developing depression (25, 26), and this risk is doubled in the presence of diabetes (Anderson et al., 2001; De Groot et al., 2001; Labad et al., 2010). Depressed mood is also associated with abdominal obesity and poor diet (Roberts et al., 2003; Dong et al., 2004; Simon et al., 2006; Luppino et al., 2010; Zhao et al., 2011; Hamer et al., 2012). A link between obesity and depression has been found in animal models of mood disorders (Lombard, 2000; Pawels and Volterrani, 2008; Dallman et al., 2003, 2005; Singh et al., 2007, 2009, 2011; Dallman, 2010; Chuang et al., 2011; Diz-Chaves, 2011; Maniam and Morris, 2012; Spence and Courbasson, 2012; Akubuiro et al., 2013; Kumar et al., 2013), suggesting that a common signaling pathway may underlie these phenotypes in both humans and animals.
There are numerous articles on the regulation of food intake, obesity, and mood. However, further exploration of the interaction among mood, food, and obesity is much needed. The aim of this review article is to highlight the complex interplay among mood, emotional state, and eating behaviors .
Central Nervous System in Regulation of Mood, Food, and Obesity
Bi-Directional Link of Food and Emotion
In humans, eating behavior is complex and is affected by both mood and emotions (Lyman, 1982; Mehrabian, 1995; Macht, 1999; Macht and Simons, 2000). However, mood and emotions are distinct. Mood is characterized by psychological arousal in the absence of obvious stimuli that can last for several minutes or longer. In contrast, emotions are short-term affective response to reinforcing stimuli. Of all emotions, a study shows that frequent emotions such as, anger and joy have the strongest influence on appetite and food choice (Macht, 1999). Behavior based findings from human studies of questionnaires, field, and clinical studies suggest an integrative five way model that predicts five different aspects of emotional eating. These five aspects include: food choice, food intake, loss of cognitive controls, food modulating emotions, and emotion-congruent modulating eating, see review by Macht (2008). Therefore, depending on the state of negative emotions or distress, emotional eating is triggered where food intake can either increase or decrease within the same individuals (Ouwens et al., 2009). Emotional state has also been connected with addiction (Parylak et al., 2011). Sensory and psychological pathways influence food choice, the quantity, and meal frequency that may not be a part of normal physiological requirement. Many psychosomatic theories of obesity suggests that obese people overeat due to inability to perceive their physiological state, hunger, and satiety and that overeating reduce emotional discomfort and anxiety (Kaplan and Kaplan, 1957; Schachter, 1968; Bruch, 1985). The internal/external theory of obesity predicts that normal eaters alter their food intake to regulate their emotion, while obese people do not (Schachter, 1968; Canetti et al., 2002). Depending on whether an eater is restrained or emotional, stress and negative emotions could be associated with both increased and decreased motivation to eat; and under those circumstances, food choice differs (Herman and Mack, 1975). Thus, emotional distress influences emotional food choice and intake.
Stress and Food Intake
There is a close interaction between food, mood, and stress (Benton and Donohoe, 1999; Oliver and Wardle, 1999; Gibson, 2006; Dallman, 2010; Bast and Berry, 2014). Stress can affect feeding behavior (Greeno and Wing, 1994; Yau and Potenza, 2013), resulting in either increased or reduced food intake depending on the types of external or psychological stressors (Oliver and Wardle, 1999; Gibson, 2006; Dallman, 2010; Yau and Potenza, 2013). Similarly, chronic stress can lead to either increased consumption of palatable and rewarding foods leading to obesity or a diminished appetite leading to weight loss (Cartwright et al., 2003; Adam and Epel, 2007; Tryon et al., 2013). Furthermore, following exposure to a stressor, studies show that intake of palatable foods reduce signs of stress and anxiety (Pecoraro et al., 2004; La Fleur et al., 2005; Maniam and Morris, 2010, 2012; Ulrich-Lai et al., 2010; Finger et al., 2011, 2012). Interestingly, stress-induced preference for palatable food is often seen in humans (Souquet and Rowland, 1989; Epel et al., 2004; Pecoraro et al., 2004; Christiansen et al., 2011; Gibson, 2012; Merali et al., 2013; Sharma et al., 2013; Sharma and Fulton, 2013; Meye and Adan, 2014; Park et al., 2014; Rho et al., 2014). Notably, this behavior is extended to animals (Dallman et al., 2003, 2005; Cottone et al., 2009). This suggests that a common neurobiological pathway maybe involved in food choice and patterns of eating behavior during stress.
Mood and Food Intake
Mood states such as anxiety and depression affect food choice and energy metabolism. Overeating and obesity is often associated with depression and anxiety in humans which has also been reported in animal models (Novick et al., 2005; Simon and Von Korff, 2006; Kloiber et al., 2007; Singh et al., 2007, 2009; Akubuiro et al., 2013; Patterson and Abizaid, 2013; Sharma and Fulton, 2013). Both endocrine and metabolic conditions are exacerbated in major depression (Mcelroy et al., 2004; Simon et al., 2006; De Wit et al., 2010; Luppino et al., 2010; Marijnissen et al., 2011). Individuals experiencing depressed moods show preference for and consume palatable “comfort foods” as a mean to alleviate their negative feelings (Macht, 2008). Although on a short-term basis, palatable foods can provide some relief from negative emotions and mood states, chronic consumption of calorically-rich foods ultimately leads to obesity which in turn promotes vulnerability to depression and anxiety (Novick et al., 2005; Simon et al., 2006; Kloiber et al., 2007; Sharma and Fulton, 2013). Conversely, there are findings showing that prolonged high-fat feeding leads to negative emotional states, increased stress sensitivity, and altered basal corticosterone levels (Sharma et al., 2012). Thus, negative emotion impacts food choice and intake that in turns affects mood in a bi-directional manner.
Interestingly, other behaviors of reduced pleasure/reward experience, anxiety-like behavior, and heightened stress-induced hypothalamic pituitary adrenal axis (HPA) activation have been found in mice. Furthermore, after exposure to chronic high-fat diet and then switching to normal chow diet, mice showed craving for sucrose, high-fat foods, and displayed enhanced anxiety-like behavior (Sharma et al., 2012). Similar findings of increased behavioral and physiological signs of depression and anxiety have been reported in humans when switched from a high-fat sugar diet to regular diet (Avena et al., 2008; Teegarden and Bale, 2008; Cottone et al., 2009; Pickering et al., 2009; Iemolo et al., 2012; Sharma et al., 2012; Blasio et al., 2013). All together, these findings suggest that chronic high-fat feeding promotes negative emotional states and potentiates condition for enhanced sensitivity to stress that leads to continuous repetitive cycles of overeating, weight gain, and depressed mood.
Food Preference and Mood
Hippocrates, father of modern medicine, said: “Let your food be your medicine, and your medicine be your food” (Prasad, 1998). Research from human trials and animal studies have shown that foods directly influence brain neurotransmitter systems which in turn has effects on mood and performance by altering the brain structure, chemistry, and physiology. Mood can also influence our food choices and expectations on the effects of certain foods can influence our sapiens. Some of those foods impacting mood are discussed below and summarized in Table 1 (Spring et al., 1982-1983; Rogers and Lloyd, 1994).
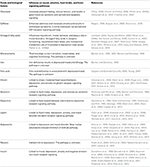 Table 1. Summary of biological factors and food influencing mood, emotions, food intake, and brain signaling pathways.
Table 1. Summary of biological factors and food influencing mood, emotions, food intake, and brain signaling pathways.Chocolate has a strong effect on mood, generally increasing pleasant feelings and reducing tension (Osman and Sobal, 2006; Parker et al., 2006b; Cartwright et al., 2007; Fletcher et al., 2007). Chocolate contains psychoactive chemicals such as andamines that stimulate the brain and result in good mood (Ottley, 2000). However, negative feelings are also associated with chocolate in some women on weight loss regimes who experience guilt after eating chocolate. The unique taste and feel from chocolate in the mouth leads to chocolate craving due to sensory factors associated with chocolate eating (Macht and Dettmer, 2006; Osman and Sobal, 2006; Parker et al., 2006b; Cartwright et al., 2007; Fletcher et al., 2007).
Caffeine, mostly consumed in the form of coffee and tea, not only has stimulant effects on enhancing alertness, vigilance, and reaction time but also increases anxiety in susceptible individuals (Acquas et al., 2002; Rossi et al., 2010). Caffeine blocks adenosine receptors in the brain and can relieve headaches, drowsiness, and fatigue. Short-term caffeine deprivation in regular users results in withdrawal symptoms (Rogers, 1995).
Omega-3 fatty acids, found in various foods can influence, mood, behavior, neuroticism, and impulse control (Van Strater and Bouvy, 2006; Conklin et al., 2007; Stahl et al., 2008). Omega-3 fatty acids play a role in major depressive disorder, bipolar disorder, schizophrenia, substance abuse, and attention deficit disorder (Young and Martin, 2003; Parker et al., 2006a; Van Strater and Bouvy, 2006; Stahl et al., 2008). Docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), both members of the omega-3 fatty acid family, contribute to the fluidity of the cell membrane, and thereby play an important role in brain development and function (Pawels and Volterrani, 2008). Low blood levels of polyunsaturated omega-3 fatty acids are associated with depression, implying a role in mood disorders (Lombard, 2000; Sanchez-Villegas et al., 2007; Antypa et al., 2012; Moranis et al., 2012; Kang and Gleason, 2013; Grosso et al., 2014).
Micronutrients, such as thiamine (vitamin B1), iron, and folic acid, play a role in emotion. Thiamine containing foods influence mood states (Benton et al., 1995). Improved thiamine status increases well-being, sociability, and overall energy levels. Insufficient amounts of thiamine are associated with impaired mood and cognitive functioning (Benton et al., 1997; Benton and Donohoe, 1999).
Iron deficiency represents one of the most common nutritional problems worldwide. Iron deficiency anemia can result in depressed mood, and problems with attention and lethargy (Benton and Donohoe, 1999).
Folic acid plays an important role in the brain. Folic acid deficiency is associated with depressed mood (Coppen and Bolander-Gouaille, 2005; Young, 2007). Psychiatric patients often run the risk of developing folic acid deficiency due to loss of appetite from anticonvulsant drugs that inhibit folic acid absorption (Ottley, 2000). Collectively, these findings suggest foods influence mood.
Mood can influence food preference (Christensen and Brooks, 2006). Choice of eating palatable foods can either lead to comfort feeling or disgust. A good example of behavioral change that is observed after taking a meal is altered mood. A general effect of meal on behavior is observed from animals to humans where hunger leads to irritability and meal intake leads to arousal and alertness. Thus, a search for food is cultivated. Once satiety sets in, sedentary and calm behaviors most likely have positive rather than negative effect on mood (Macht and Simons, 2000; Macht et al., 2003; Macht and Dettmer, 2006; Macht, 2008). A potential internal information route on emotional behavior was first recognized in 2001 where nutrients from gut were relayed to the brain by the vagus nerve affecting emotions (Zagon, 2001). However, the relationship of emotions, physiological arousal, and mood in a given situation is significantly dependent upon on the subject’s motivational state (Reid and Hammersley, 1999) and the individual’s personality trait of neuroticism that interacts with mood and response to emotional stimuli (Dess and Edelheit, 1998).
The pathogenesis of both mood and metabolic disorders during obesity can be triggered by certain diets (Wallin and Rissanen, 1994; Sanchez-Villegas and Martinez-Gonzalez, 2013). Diets like Western diets that are rich in saturated fat and low in poly-unsaturated and mono-unsaturated fatty acids tend to increase the incidences of depression (Peet et al., 1998). On the other hand, diet like the Mediterranean diet appears to reduce depression (Sanchez-Villegas and Martinez-Gonzalez, 2013; Sanchez-Villegas et al., 2013). Furthermore, many reports show the increased incidence of depression on diets that lack omega-3 polyunsaturated fatty acids (PUFA) and that depression is reduced when intake of PUFA is increased in both humans (Lin and Su, 2007; Sanchez-Villegas et al., 2007; Oddy et al., 2011; Park et al., 2012a) and rodents (Moranis et al., 2012; Park et al., 2012b). Besides mood changes, high fat diets promote increased weight gain, visceral adipose tissue, larger waist circumference, and more cardiovascular disease mortality (Schulze et al., 2006; Molenaar et al., 2009; Romaguera et al., 2009, 2010; Mozaffarian et al., 2011; Estruch and Salas-Salvado, 2013; Nazare et al., 2013). The accumulation of adipose tissue in abdominal stores leads to several complications of obesity including insulin resistance leading to metabolic syndrome (Despres et al., 2006; Tchernof and Despres, 2013). These changes also lead to neurobiological impairments affecting mood disorders such as depression and anxiety (Weber-Hamann et al., 2002; Van Reedt Dortland et al., 2013a,b). It is believed that increased circulating plasma fatty acids such as palmitic acid enters the brain and impairs neurological function (Tsuboi et al., 2013). Palmitic acid impairs leptin and insulin receptor signaling in the hypothalamus and promotes weight gain (Benoit et al., 2009; Kleinridders et al., 2009). Under these circumstances, obesity is promoted, as well as a negative emotional state. In addition, leptin and insulin have been noted to influence mood (Gonder-Frederick La et al., 1989; Lu et al., 2006; Lu, 2007; Zeman et al., 2009; Ryan et al., 2012).
Furthermore, several studies have shown humans on high fat diet manifest mood disorders like depression that correlates positively with high serum palmitate (Tsuboi et al., 2013). Similarly, rats on high fat diet display increased anxiety-like behavior, altered body weight, plasma insulin, leptin, and glucose levels when compared to rats on iso-caloric olive oil high fat diet that show no changes in body weight, glycaemia, leptin, and insulin levels (Hryhorczuk et al., 2013). Thus, saturated fats stimulate HPA disturbances and/or inflammation, leading to anxiogenic-like behavior in animals and depression in humans. All together these findings suggest an association between certain foods and improved mood.
Psychiatric and Eating Disorders
The Diagnostic and Statistical Manuals of Mental Disorders (DSM-5), which was developed by the American Psychiatric Association in 1994, reported disturbed eating behaviors in psychiatric disorders (American Psychiatric Association, 2013). In humans, melancholic depression is associated with hypercortisolism, anhedonia, hypophagia, and weight loss (Fisher et al., 1997; Krishnan and Nestler, 2008; Ulrich-Lai and Herman, 2009; Hammack et al., 2010; Carroll et al., 2012; Hryhorczuk et al., 2013; Patterson and Abizaid, 2013; Schellekens et al., 2013b). In contrast to atypical depression, the most common forms of depression are characterized by reduced hypothalamic pituitary adrenal axis (HPA) activity, increased appetite, carbohydrate craving, and weight gain (Juruena and Cleare, 2007). Those with abdominal obesity are associated with hyperactive HPA axis due to an elevated response to corticotrophin releasing hormone (CRH) stimulation and increased stimulated response to stress (Pasquali, 2012).
Altered serum cortisol level is associated with depression (Parker et al., 2003; Raison and Miller, 2003; Stetler and Miller, 2011). Altered cortisol, HPA axis, and food intake have been associated with depression (Ulrich-Lai and Herman, 2009; Dallman, 2010; Schellekens et al., 2012a). The neuronal pathways that regulate food intake, and circuitries that act via the HPA axis are implicated in a complex two-way relationship of three concepts between mood, food, and eating behavior (Figure 1) (Kyrou and Tsigos, 2009; Ulrich-Lai and Herman, 2009; Dallman, 2010; Schellekens et al., 2012b, 2013b). It is noted that there is an overlap in neural circuitry of food intake and stress that likely reinforces a link between stress and feeding behavior (Maniam and Morris, 2012). These overlapping circuitries of HPA axis modulating feeding behavior and stress converge on corticosterone hormone producing neurons in the paraventricular nucleus (PVN). Thus, elevated glucocorticoid and a dysfunctional HPA axis are common to both depression and obesity.
Glucocorticoids exert multiple effects on metabolic, endocrine, immune, and behavioral functions. Glucocorticoids regulate reward and emotional processes via their receptors in midbrain and limbic circuits (Arnett et al., 2011; Solomon et al., 2012; Hryhorczuk et al., 2013; Patterson and Abizaid, 2013; Wang et al., 2013). Glucocorticoids not only act peripherally to maintain energy homeostasis but also centrally to modulate HPA activity, emotional, and behavioral effects of stress (Fedoroff et al., 2003; Figueiredo et al., 2003). Under physiologic acute stress, the HPA axis is activated, and glucocorticoids are released. This leads to a major restoration of energy balance by increasing insulin, increasing motivation for palatable food (Piazza and Le Moal, 1997; Dallman et al., 2006; Dallman, 2010), and mobilizing stored energy toward central stores that leads to obesity (Mann and Thakore, 1999). Thus, obesity and mood disorder are linked via the HPA axis. In rodents, chronic corticosterone exposure leads to increased glucocorticoid receptor (GC) expression in fore-brain and basolateral amygdala that results in depressive-like, anxiety-like behaviors, and increased locomotors (Wei et al., 2004; Boyle et al., 2005, 2006). Therefore, these findings suggest that a deficit in glucocorticoid signaling in distinct brain regions may play a role in affective disorder.
Obesity and Mood
Obesity increases incidence of anxiety and mood disorders (Simon et al., 2006). Stress induced overeating and obesity is also associated with major depression in humans (Novick et al., 2005; Simon et al., 2006; Kloiber et al., 2007). Individuals under chronic stress tend to have more visceral fat due to excessive systemic cortisol levels (Brown et al., 2004; Adam and Epel, 2007; Kyrou and Tsigos, 2009). In all, there appears to be a good association between hypercortisolemic depression, abdominal fat accumulation (Weber-Hamann et al., 2002), decreased glucocorticoid-mediated negative feed back, and increased corticotropin releasing hormone (CRH) release from the paraventricular nucleus (PVN) (Holsboer, 2000). Furthermore, major depression in adolescence is linked to a higher risk for obesity in adulthood (Richardson et al., 2003). It is also noted that metabolic conditions are exacerbated in depression and vice versa (Mcelroy et al., 2004; Simon et al., 2006; De Wit et al., 2010; Luppino et al., 2010; Marijnissen et al., 2011). Like-wise, stress significantly impacts food intake in both humans and animals, thereby promoting metabolic disturbances (Block et al., 2009; Dallman, 2010; Maniam and Morris, 2012). Overeating can also be considered to be analogous to drugs of use because it reflects an addiction where individuals become physically and psychologically dependent on foods rich in fat and sugar (Avena et al., 2008, 2009; Barry et al., 2009; Parylak et al., 2011; Allen et al., 2012; Davis, 2013). Reports also show that with intake of palatable rewarding food, acute stress responses are reduced (Dallman et al., 2003; Lutter and Elmquist, 2009; Chuang et al., 2011; Kumar et al., 2013), thereby showing the potential of “comfort eating” in stress relief. All together these findings suggest that there is a reciprocal link in mood disorder and obesity.
Peripheral System in Regulation of Mood, Food, and Obesity
The gut-brain axis mediates the communication between brain and gut when it comes to appetite, satiety, and energy homeostasis (Cummings and Overduin, 2007; Ahima and Antwi, 2008; Blevins and Baskin, 2010; Gibson et al., 2010; Suzuki et al., 2010, 2012). Furthermore, peripheral hormones have also been reported to regulate mood, food intake, and obesity (Tschop et al., 2000; Nakazato et al., 2001; Olszewski et al., 2008; Blevins and Baskin, 2010; Suzuki et al., 2010; Andrews, 2011b; Dickson et al., 2011; Egecioglu et al., 2011; Skibicka and Dickson, 2011; Overduin et al., 2012; Perello and Zigman, 2012; Karra et al., 2013). Gastrointestinal signals such as cholecystokinin (CCK), bombesin, glucagon eneterostatin, insulin, resistin, somatedin, cyclohistiyl-proline, leptin, amylin, and apolipoprotein A-IV are all known to reduce food intake. The exception is ghrelin, which increases food intake. Several peripheral factors that engage the CNS in a bi-directional manner and influence mood and food intake are summarized in Table 1 and discussed below.
Ghrelin
A gut orexigenic hormone ghrelin is synthesized in the stomach and acts centrally to mediate increased food intake via central pathways (Kojima et al., 1999, 2004; Tschop et al., 2000; Nakazato et al., 2001; Andrews, 2011a; Diz-Chaves, 2011). The hypothalamus in the brain directly senses peripheral ghrelin and modifies the energy status (Schaeffer et al., 2013). Studies support that ghrelin reaches the brain via the vagus afferents to the nucleus solitary tract (NST), which further projects to the arcuate nucleus of the hypothalamus (Asakawa et al., 2001; Date et al., 2002; Williams and Mobarhan, 2003). Ghrelin activates downstream signaling via the hormone secretagogue receptor (GSH-R1a) where it is ubiquitously expressed in multiple brain regions and in peripheral tissues. Due to multiple sites of GSH-R1a expression, it is not surprising that ghrelin performs many other biological activities of growth hormone secretion, glucose and lipid metabolism, and gastrointestinal motility. However, other properties of GHS-R1a allowing dimerization with multiple G-protein coupled receptors suggest the likelihood of cross talk between many other neuropeptide systems of serotonin and dopamine (Schellekens et al., 2013a,b). Thus, ghrelin has the potential to engage multiple neuropeptide systems in mood, food, and obesity.
The ghrelinergic system also mediates the non-homeostatic hedonic rewarding and motivational aspects of food intake via mesolimbic dopaminergic circuitry (Dickson et al., 2011; Egecioglu et al., 2011; Skibicka et al., 2011; Perello and Zigman, 2012). Studies support ghrelin’s involvement in stress mediated food reward behavior (Perello et al., 2010; Kumar et al., 2013; Chuang et al., 2011; Diz-Chaves, 2011). Numerous studies provide a link between ghrelin and affective disorders, such as depression and anxiety (Schanze et al., 2008; Barim et al., 2009; Kluge et al., 2009). Ghrelin also alleviates depression (Kluge et al., 2011). All together these studies suggest that the ghrelinergic system is an attractive system to target stress associated metabolic and mood associated eating disorders in obesity.
Serotonin
Serotonin has numerous functions besides regulating mood that includes regulation of sleep, appetite, and impulse control (Steiger, 2004; Daubert and Condron, 2010; Nordquist and Oreland, 2010; Mosienko et al., 2012). Serotonin levels from the gut and alimentary canal constitutes about 80–90% of the human body’s total serotonin and not in the brain. This is surprising, as serotonin dictates most of our mood and happiness (Wurtman and Wurtman, 1989; Benton and Donohoe, 1999). Central serotonin pathways participate in the regulation of mood and modulate meal patterns in terms of quality and quantity. Neurotransmitter release of serotonin from serotonergic neurons in the brain is governed by food intake (Shabbir et al., 2013). The essential amino acid tryptophan that comes from food is the precursor for serotonin synthesis (Prasad, 1998). Ingestion of carbohydrates increases the plasma ratio of tryptophan to other large neutral amino acids leading to increased serotonin synthesis in the brain and alleviating depression. Such is the case for carbohydrate craving during depression that often leads to obesity and vice versa (Pepino et al., 2009; Shabbir et al., 2013). This is observed during stress, winter depression, or in people trying to give up smoking. Nicotine increases brain serotonin secretion and its withdrawal leads to depression (Wallin and Rissanen, 1994; Wurtman and Wurtman, 1996). Brain serotonin plays a role in the pathophysiology of depression, as treatments with serotonin potentiating drugs alleviates depression in seasonal affective disorder (Wurtman, 1993). Based on these findings it has been suggested that the excessive carbohydrate intake by patients with premenstrual syndrome (PMS) and seasonal affective disorder (SAD) relieves the depressive symptoms via an increased central serotonergic activity (Cizza et al., 2005; Miller, 2005). A diet rich in carbohydrates can relieve depression and elevate mood (Wurtman and Wurtman, 1989; Benton and Donohoe, 1999). Furthermore, research has shown that dieters tend to become depressed as the serotonin levels are reduced due to decreased carbohydrate intake (Huether et al., 1997). Thus, these studies imply that certain foods are strong mood regulators.
Leptin
Low leptin levels have been found to be associated with human depression and depression-like behaviors in rodents (Kraus et al., 2001; Lu et al., 2006; Guo et al., 2012; Lawson et al., 2012). Antidepressant-like effect of leptin in leptin insufficiency or leptin resistance suggests the hormone contributes to altered mood (Lu, 2007). Increased visceral fat and dyslipidemia are associated with several endocrine and metabolic changes that link to CNS control of emotional states and mood (Hryhorczuk et al., 2013). As an endocrine gland, adipose tissue secretes numerous peptide hormones that target the brain and peripheral tissues to regulate metabolism and behavior. Leptin circulates in proportion to fat mass (Maffei et al., 1995). Leptin impacts several physiological processes such as appetite, energy expenditure, and neuroendocrine function. The hormone has also been linked to human depression and has been shown in rodents to have antidepressant and anxiolytic effects (Asakawa et al., 2003; Liu et al., 2010; Yamada et al., 2011; Lawson et al., 2012). Nevertheless, there are conflicting findings of leptin levels and depression, which are discussed below.
Major depressive disorder (MDD) has been shown to be associated with lower plasma leptin levels when compared to healthy controls (Kraus et al., 2001; Atmaca et al., 2002, 2008; Westling et al., 2004; Jow et al., 2006). On the other hand, there are reports showing increased plasma leptin levels in depression (Kraus et al., 2002; Esel et al., 2005; Schilling et al., 2013), gender specific increased leptin levels in women with depressive disorder (Rubin et al., 2002; Esel et al., 2005; Zeman et al., 2009), as well as no changes of leptin by antidepressant treatment (Esel et al., 2005). In depressed individuals suffering from loss of appetite, plasma leptin levels do not differ from those of healthy controls (Deuschle et al., 1996). In another study, it was found that higher serum leptin was associated with atypical depressive patients with increased appetite (Gecici et al., 2005). In older men, a combination of elevated visceral fat and high leptin levels was associated with depression (Milaneschi et al., 2012), and high leptin correlated positively with depressive symptoms in patients with type 2 diabetes (Labad et al., 2012). Thus, these reports suggest more studies are required to draw a better conclusion regarding the role of leptin in human depression.
Interestingly, rodent studies have provided the most conclusive findings. Leptin modulates the HPA axis and mice that lack leptin (obese ob/ob mice or its leptin receptor (obese db/db mice) show increased depression-like behavior (Collin et al., 2000; Asakawa et al., 2003; Lu et al., 2006; Finger et al., 2010; Liu et al., 2010; Sharma et al., 2010; Yamada et al., 2011; Guo et al., 2012, 2013). Furthermore, leptin deficient ob/ob mice have elevated corticosterone that can be reduced by leptin replacement (Garthwaite et al., 1980; Arvaniti et al., 2001). In contrast, chronic unpredictable mild stress in rats activates the HPA axis and leads to depressive-like behaviors that correlate with decreased serum leptin levels (Ge et al., 2013). Leptin receptors (LepRb) in midbrain and forebrain loci that affect emotional processes are targeted by leptin. Genetic deletion of LepRb in the hippocampus results in a depression-like phenotype, which is reduced by leptin administration to the hippocampus thereby showing an antidepressant effects (Asakawa et al., 2003; Lu et al., 2006; Finger et al., 2010; Liu et al., 2010; Guo et al., 2013). Loss of LepRb specifically in glutamatergic neurons of the forebrain elicits depressive-like behavior without affecting anxiety (Guo et al., 2012). Stress-induced dopamine release is also associated with high leptin (Burghardt et al., 2012). Leptin activates dopamine neurons in the VTA of the midbrain reducing dopamine neuronal firing and increases dopamine availability (Fulton et al., 2006; Hommel et al., 2006). Selective deletion of LepRb from midbrain dopamine neurons results in increased anxiety-like behavior, but not depressive-like behavior (Liu et al., 2011). LepRb signaling in limbic and prefrontal nuclei mediates the antidepressant action of leptin. In contrast, leptin in dopamine neurons of the ventral midbrain and in central nucleus of the amygdala leptin signaling exerts the anxiolytic actions of leptin. Thus, leptin signaling in different brain regions exerts different physiological behaviors.
In conditions of central obesity that favors insulin resistance and type 2 diabetes, leptin sensitivity is diminished. Leptin resistance is associated with high plasma leptin levels and defective LepRb signaling. These states are characteristic of obesity and increase the risk for mood disorders (Myers et al., 2012). Mice made obese by a high fat diet intake show reduced sensitivity to effects of leptin and antidepressant actions of leptin when compared to low-fat diet treated controls (Yamada et al., 2011). Further, leptin insensitivity exacerbates HPA dysregulation in obesity (Komorowski et al., 2000; Collura et al., 2009) and thereby enhances the mass of dysfunctional central adipose stores in a cortisol-dependent manner. Leptin resistance has been reported to be associated with the mid brain VTA where mesolimbic DA neurons reside (Matheny et al., 2011). Leptin resistance appears to affect multiple neural and endocrine pathways including hippocampal, mesolimbic dopamine pathways, and HPA activity ultimately affecting emotions and mood. Thus, these studies provide evidence of leptin related mechanisms underlying depression in obesity.
Adiponectin
Low levels of another adipose-derived hormone, adiponectin, has been implicated in energy homeostasis, metabolic disturbances, insulin resistance (Kennedy et al., 2006; Hanley et al., 2007; Turer and Scherer, 2012; Hryhorczuk et al., 2013) and recently, depression in humans (Arita et al., 1999; Cnop et al., 2003; Ryo et al., 2004; Leo et al., 2006; Narita et al., 2006; Hanley et al., 2007; Weber-Hamann et al., 2007; Yilmaz, 2008) and rodents (Maeda et al., 2001; Milan et al., 2002; Delporte et al., 2004; Ye et al., 2007). Changes in adiponectin levels are secondary to metabolic disturbances in obesity (Morrison et al., 2011; Doumatey et al., 2012). There are conflicting reports of either positive or negative associations of adiponectins levels with mood disorder (Yilmaz, 2008; Zeman et al., 2009; Jeong et al., 2012; Wilhelm et al., 2013), or no changes in patients with major depressive disorder or with antidepressants (Lehto et al., 2010; Jeong et al., 2012). Mice exposed to chronic social defeat recapitulate the low levels of adiponectin, stress-induced depressive-like behaviors, and impaired HPA axis (Liu et al., 2012). Interestingly central administration of adiponectin has antidepressant effects (Liu et al., 2012). Thus, a link between plasma adiponectin levels and depression is observed in mice. In contrast, humans show more ambiguous results depending on the type of depressive disorder, sex, and treatment.
Resistin
Adipocyte-derived resistin is linked to insulin resistance in rodent models of depression-like behavior while in humans, the role of resistin is less defined (Schwartz and Lazar, 2011; Hryhorczuk et al., 2013). In genetic and diet induced obese mice circulating resistin levels are elevated (Steppan et al., 2001). In contrast, resistin is down regulated in human obesity (Way et al., 2001; Degawa-Yamauchi et al., 2003; Owecki et al., 2011; Sadashiv et al., 2012). However, there is one study that shows a positive correlation between resistin levels and atypical depression (Lehto et al., 2010). In human depression, however, resistin levels positively correlate with salivary cortisol (Krsek et al., 2004; Silha et al., 2004; Weber-Hamann et al., 2007). Conversely, resistin levels are lower in patients receiving antidepressant treatment who have remitted from depression (Weber-Hamann et al., 2007). Thus, these studies imply that resistin plays a role in affecting mood.
Insulin
From a recent systematic review and meta-analysis there appears to be a significant cross-sectional association between depression and insulin resistance (Kan et al., 2013) and there is a bi-directional association between diabetes and depressed mood. Depression is associated with pre-diabetes insulin resistance (Anderson et al., 2001; Kan et al., 2013) and obesity (Hamer et al., 2012). However, there exists a weak association of insulin resistance and depression (Adriaanse et al., 2006; Platt et al., 2013; Shen and Bergquist-Beringer, 2013). High fat diet intake impairs the hypothalamic insulin receptor signaling (De Souza et al., 2005; Kim and Feldman, 2012) and reduced hypothalamic insulin signaling promotes weight gain and negative emotional states (Gustafson et al., 1999; Koponen et al., 2008; Akbaraly et al., 2009; Almeida et al., 2009; Benoit et al., 2009; Kleinridders et al., 2009; Pulkki-Raback et al., 2009; Platt et al., 2013). Intranasal insulin ameliorates self-reported mood, reduce cortisol levels, and visceral obesity (Benedict et al., 2004; Chapman et al., 2013). Further treating patients with major depressive disorder and abdominal obesity, the insulin-sensitizing drug pioglitazone shows reduced sign of depression, anxiety, and reduced insulin resistance (Kemp et al., 2012).
In rodents, reduced insulin receptor signaling impacts mood when placed on a long-term 30%kcal fat diet that shows anxiolytic effects (Marks et al., 2009). Similarly, rosiglitazone administered to normal chow-fed mice and rats show an antidepressant action in behavioral despair tests (Eissa Ahmed et al., 2009; Ryan et al., 2012). Antisense RNA targeting the insulin receptor in rats results in increased depression-like behavior and anxiety-like behavior (Grillo et al., 2011). By and large, these results suggest that insulin signaling is involved in mood. However, further studies are required to determine whether intranasal insulin has antidepressant effects in depressed individuals and, if so, whether this action is maintained in obesity.
To summarize, food intake is regulated by the peripheral and central system that are engaged in a bi-directional manner. Peripheral signals mostly modulate satiety and indicate adiposity signal to the brain. Ghrelin is the only peripheral hormone that induces hunger but interestingly it is also involved in mood and hedonic aspects of food intake. There are several brain regions involved in food intake that overlaps brain areas involved in drugs of abuse and reward. Overlapping brain regions of reward, mood, and food intake suggests that molecular changes in these regions may provide further insights in to distinct and overlapping pathways that could aid in understanding clinical treatments of comorbidity of mood disorder, overeating, and obesity.